Energy – Sources, Conversion and Storage 2
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
What are the 3 main steps involved in processing of crude oil into desired products?
Frational Distillation
Cracking of petroleum
Reforming of petrol
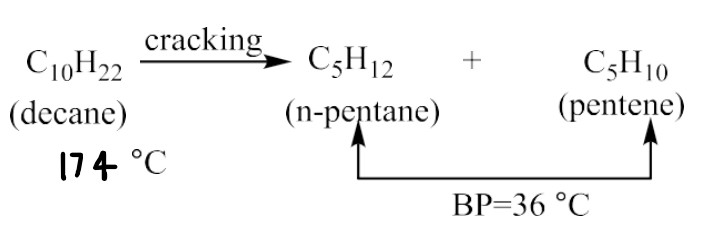
What is Cracking of petroleum?
Decomposition of bigger hydrocarbons into simpler low boiling hydrocarbons of low molecular weight.
What is the need for cracking in the petroleum industry?
To produce extra petrol: There is surplus of heavier petroleum fractions. Heavy fractions in less demand can be cracked to produce extra petrol.
To improve the quality of ‘straight-run’ gasoline, it has to be properly blended. The characteristics of gasoline obtained by cracking are far more superior to the straight run gasoline.
To produce alkenes: Cracking always produces alkenes that can be used to make many useful organic chemicals.
What are the methods for cracking?
Thermal Cracking where heavy oils are subjected to high temperature and pressure when the bigger hydrocarbons molecules break down to give smaller molecules.
Catalytic Cracking: Procedure involves a suitable catalyst like aluminium silicate Al2(SiO3)3 or alumina Al2O3
What are the reaction conditions for Moving or Fluidized-Bed catalytic cracking?
Feed Stock: Heavy Oil
Catalyst used: y-type zeolite activated with a rare earth oxide (12.5% Al2O3 + 87.5% SiO2)
Temperature: 450-550∘C
Pressure: 1 atm
Yield: 70-80%
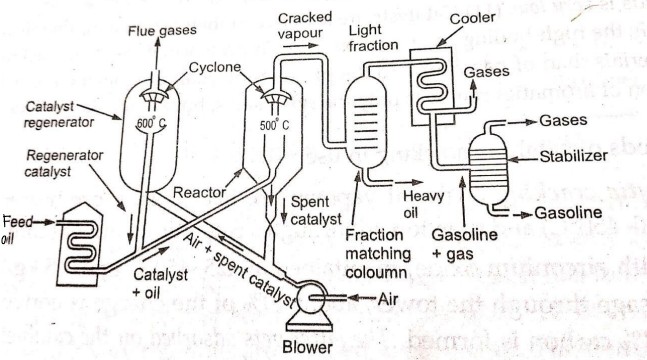
Explain the procedure of Fluidized-Bed Catalytic Cracking
Solid catalyst is finely powdered (fluid-like behaviour) which is circulated in gas stream
Heavy oil vapours + Fluidized catalyst mixed forced into large reactor bed where cracking of heavier into lighter molecules occurs.
Centrifugal separator (Cyclone) (top of reactor) allows cracked oil vapours to pass into fractionating column, retains catalyst in reactor itself.
Spent catalyst is continuously transported to regeneration chamber through air stream.
Carbon on catalyst particles is burnt off in regeneration chamber
Regenerated catalyst is transported back to cracking chamber together with feed stock.
What are the advantages of Fluidized-bed catalytic cracking?
The yield of petrol is higher.
The quality of petrol produced is better.
Fuel after cracking has higher percentage of branched chain.
Fuel after cracking has higher percentage of aromatics.
Highly controlled process.
No wastage of catalyst
What are the disadvantages of fluidized-bed catalytic cracking?
Rapid mixing of fuel and catalyst may lead to non-uniform residence time ( mix of over-breakdown and under-breakdown)
Agglomeration of fine particles. (clumps reduce flow)
Fine particles of catalyst may be carried away during pressurized flow. (contamination of product, waste of catalyst)
Erosion of pipes
What is reformation of petrol?
Conversion of straight chain hydrocarbons in petrol into branched chain, cylic, aromatic hydrocarbons, resulting in upgradation of quality of petrol.
What is Octane number of a fuel?
The octane number of a fuel is the % by volume of iso-octane in a mixture of iso-octane and n-heptane that has the same knocking characteristics as the test fuel under the same operating conditions.
Octane number depends on the structure of hydrocarbons:
Straight chain alkane < Branched alkanes < Alkenes < Cycloalkanes < Aromatics [Knocking Tendency]
![<p>The octane number of a fuel is the % by volume of iso-octane in a mixture of iso-octane and n-heptane that has the same knocking characteristics as the test fuel under the same operating conditions.</p><p>Octane number depends on the structure of hydrocarbons:</p><p>Straight chain alkane < Branched alkanes < Alkenes < Cycloalkanes < Aromatics [Knocking Tendency]</p>](https://knowt-user-attachments.s3.amazonaws.com/174b1930-2958-4ea7-a2e8-2bf3e8797249.jpg)
How reformation improves the quality of petrol?
The octane number for straight chain hydrocarbons is low.
For branched chain, cyclic and aromatic hydrocarbons, the octane number is high.
Thus reformation converts the low octane number petrol into high octane number petrol.
What are the reaction conditions for reformation of petrol?
Feed Stock: Pre-processed gasoline
Catalyst: Platinum supported on alumino-silica
Temperature: 460-530∘C
Pressure: 35-50 atm
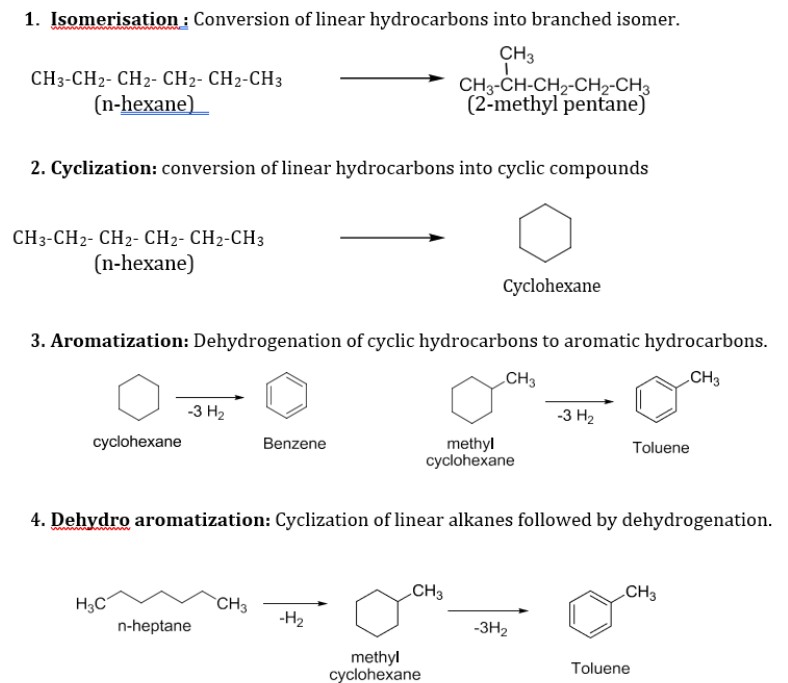
List the main Reformation reactions and explain each
Sir’s Answer:
Isomerization: n-hexane to 2-methylpentane
Dehydrogenation:
Cyclohexane → Benzene (-3H2)
Methylcyclohexane → Toluene
Dehydrocyclization: n-heptane → methylcyclohexane (-H2) → Toluene (-3H2)
Hydrocracking: n-decane → n-pentane (Pt,H2)
What are some Disadvantages of Non-Renewable energy?
Once a non renewable energy source is used up it cannot be replaced again.
Non renewable energy source are highly polluting sources and increase the greenhouse gases.
The exposure to non renewable energy sources has increased the level of pollution.
The rise in temperature due to greenhouse gas accumulation.
What is a Battery?
Electrochemical device which consists of 2 or more galvanic cells connected in series/parallel/both which produces electricity by means of redox chemical reactions.
List Basic components of battery and their roles
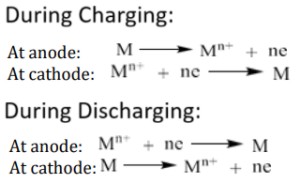
Anode: Electro-active material at anode is oxidised and liberates electrons to the external circuit. M → Mn+ + ne-
Cathode: Electro-active material at cathode is reduced and accepts electrons from the external circuit. Mn+ + ne- → M
Electrolyte: Provides medium for transfer of ions between anode and cathode.
Separator: Separates anode and cathode compartments to prevent internal short circuiting.
What properties make up good anode electro-active material?
Ease of oxidation (i. e., low reduction potential)
Capacity to deliver high Columbic output
Good conductivity
High stability
Ease of fabrication
What properties make up good cathode electro-active material?
High reduction potential
High resistant to the electrolyte
What properties make up good Electrolyte?
It is commonly a solution (or slurry) of an acid, alkali or salts having good ionic conductivity.
Safe to handle
Non-reactive with the electrode
What properties make up good Separator?
It is an electrical insulator.
Eg: Cellulose, cellophane, nafion membranes
What is the Classification of Batteries?
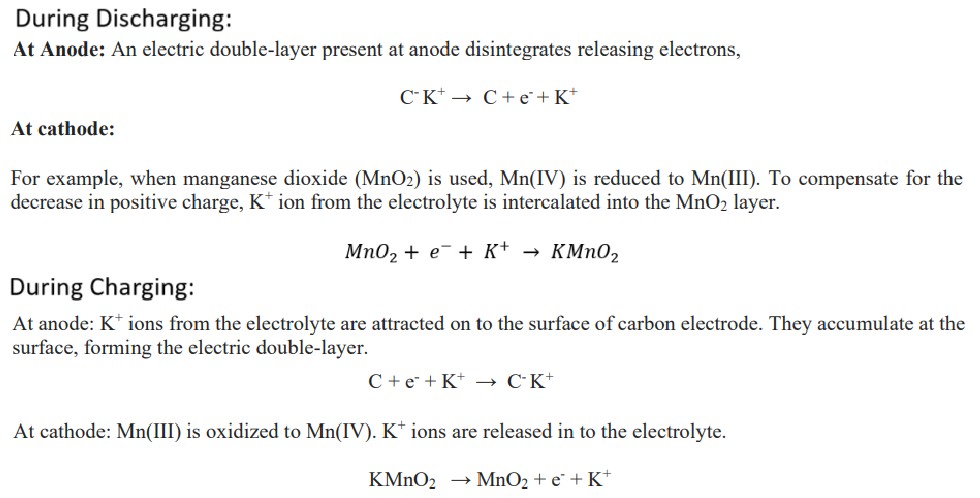
Primary Batteries: Non-rechargeable batteries. Eg: Zn-MnO2 battery, Li-MnO2 battery
Secondary batteries: These are rechargeable batteries. Eg: Nickel-cadmium battery, Lithium-ion battery
Reserve batteries: In these batteries, one of the active components (e.g. electrolyte) of the battery is separated from the rest of the components. It is assembled just before the use. Eg: Mg-AgCl and Mg-CuCl batteries; both can be activated by adding water.
How does battery work?
Discharging: Oxidation takes place at the anode and reduction takes place at the cathode. The reactions are spontaneous. Chemical energy is converted into electrical energy. It acts as a galvanic cell during discharge.
Charging: Reverse reactions take place. The reverse reactions are non-spontaneous reactions. The battery is connected to an external DC power supply. Electrical energy is converted in to chemical energy. During charging it acts as a electrolytic cell.
List characteristics of Batteries
Capacity
Power Density
Energy Efficiency
Cycle Life
Define Capacity of Battery
Capacity is a measure of the amount of electricity that may be obtained from the battery. It is expressed in Ah (ampere hours).
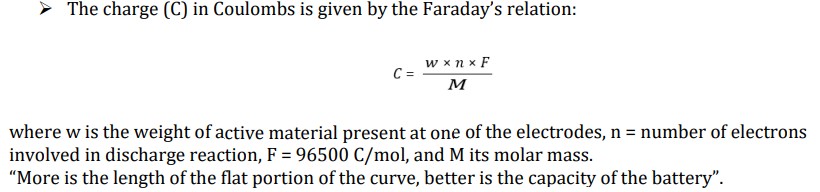
In a discharge curve, what feature of the graph shows that a battery has higher capacity?
A longer flat portion means the battery can maintain a steady voltage for a longer time, meaning it has more energy stored.
This indicates that the battery can supply usable power for a longer duration.
“More is the length of the flat portion of the curve, better is the capacity of the battery”
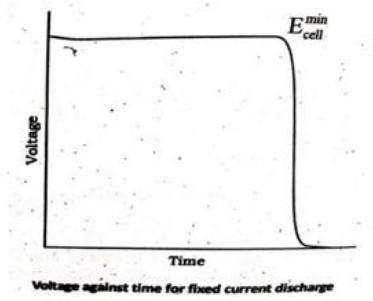
Define Power density of a battery
Power density measures how quickly a battery can deliver power per unit weight — higher power density means more power in a lighter battery.
It is the power per unit weight (or volume) of the battery. It may be expressed in W/kg
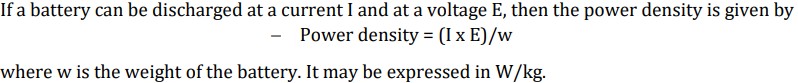
Define Energy efficiency of a battery
Define Cycle Life of a battery
It is the number of discharge–charge cycles possible in a rechargeable battery before failure occurs. A good battery must have high cycle life.
What is the Principle of Lithium-ion battery?
Li-ion batteries are secondary batteries.
During the charge and discharge processes, Lithium ions are inserted or extracted from interstitial space between atomic layers within the active material of the battery.
Exactly, in a spontaneous reaction, the lithium ions naturally move from the anode to cathode during discharge.
The Li-ion is transferred between anode and cathode through an Electrolyte.
What is the Construction of Lithium-ion battery?
Anode: Lithiated Graphite Carbon
Cathode: Lithium cobalt oxide (LiCoO2)
Separator: Polypropylene membrane
Electrolyte: Lithium salt in an organic solvent (LiPF6 in ethylene carbonate)
Cell representation: Li/Li+,C/LiPF6 in ethylene carbonate/Li-CoO2
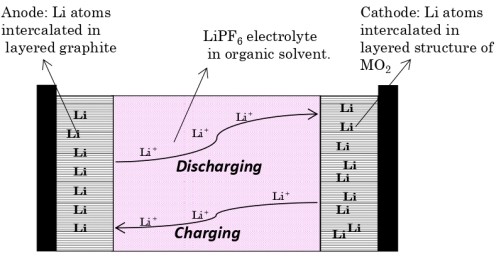
How does Lithium-ion battery work?
The traditional batteries are based on galvanic action but Lithium ion secondary battery depends on an "intercalation" mechanism.
This involves the insertion of lithium ions into the crystalline lattice of the host electrode without changing its crystal structure.
What are the Reactions involved in working of Lithium-ion battery?
At Anode:
During discharging of battery, Lithium atoms present in graphite layer (one Li atom present in every 6C atoms) are oxidized, liberating electrons and lithium ions.
Electrons flow through the external circuit to cathode and Lithium ions flow through the electrolyte towards cathode.
At Cathode:
Cobalt is reduced and Lithium atoms and are inserted into the layered structure of CoO2.
The lithium ion is inserted and exerted into the lattice structure of anode and cathode during charging and discharging.
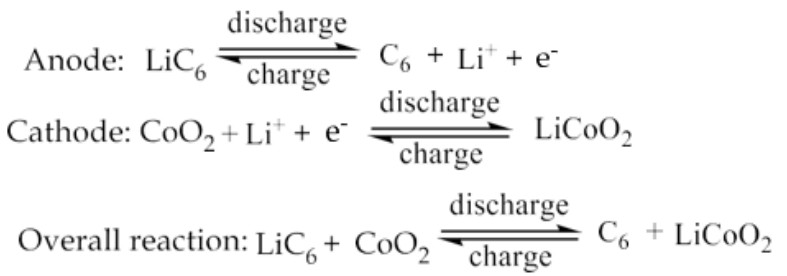
How do electron flows differ in a Li-ion battery during discharge and charging?
During discharge current flows through external circuit and light glows.
During charging, no electrons flow in the opposite direction.
What are the Advantages of Lithium-ion battery?
They have High Energy Density than other rechargeable batteries.
They are Less weight.
They produce High Voltage at about 3.7 V as compared with other batteries.
They have improved safety, i.e. more resistance to Overcharge.
No liquid electrolyte means they are immune from leaking.
What is the Construction of Sodium ion battery?
Anode: Anode material used in SIB is Hard Carbon. (larger interlayer spacing because Sodium is larger than Lithium)
Cathode:
Cathode material is Sodium inserted in layered metal oxide. CoO2 or MnO2 (Eg. LiCoO2).
Recently Mixed metal oxides like NaNi0.88Mn0.06Al0.06O2, showing better performance are used instead of single metal oxide.
Separator: Polypropylene membrane
Electrolyte: Electrolyte used is a sodium salt like NaPF6 dissolved in binary organic solvent mixture such as ethylene carbonate-dimethyl carbonate.
Note: Lithium easily forms alloy with Aluminium whereas Sodium does not. Thus in SIB, more cheaper Al foil is used as current collector. Whereas, costly Copper metal foil is used as current collector in LIB.
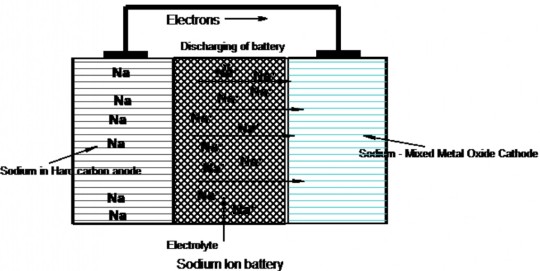
Explain Working of Sodium ion battery
What are some Advantages and Disadvantages of Sodium-ion battery?
Advantages:
Cheaper
Li mining is not environment friendly
Li is not found easily
Disadvantages:
Na ion is less efficient because of overcharging
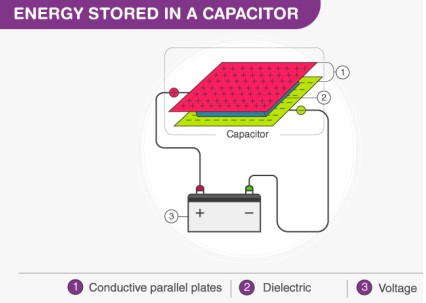
What is the basic principle of Capacitor?
A capacitor stores energy electrostatically by accumulating ions from an electrolyte on the surface of two electrodes.
No chemical reaction occurs.
C=Q/V
Differentiate between Batteries and Capacitors and Supercapacitors
Batteries
Store energy through redox (chemical) reactions.
Reactions occur throughout the bulk of the electrode.
Provide high energy but low power density (slow charge/discharge).
Capacitors
Store energy electrostatically → allows for faster charging/discharging
Provide high power, low energy.
Fast charge/discharge.
Supercapacitors/Ultracapacitor or Alectrochemical capacitor.
Bridge the gap between conventional capacitors and batteries.
They are capable of providing higher energy with higher power density.
A supercapacitor stores electrical energy like a capacitor but holds much more charge.
They have the ability to store exceptionally large amounts of electrical energy compared to conventional capacitors often thousands of times more.
While a typical capacitor might store microjoules of energy, supercapacitors can store several joules to kilojoules of energy in a compact form.
Explain Construction of Supercapacitor
A supercapacitor has four main components:
2 Electrodes
Made of high surface area materials such as:
activated carbon
carbon nanotubes (CNTs)
graphene
Surface area can exceed 2000 m²/g.
Large electrode-electrolyte interface surface area → more charge storage.
Electrolyte
Types:
Aqueous (water-based)
High conductivity
Low voltage limit (~1.2 V) due to water decomposition
Organic electrolytes
Lower conductivity
Higher operating voltage (2.5–3 V)
Ionic liquids
Very stable
High voltage capability
More expensive
Separator
A porous insulating membrane.
Prevents the two electrodes from touching (short circuit).
Allows ions to pass freely.
Current Collectors
Metallic conductors attached to electrodes to carry electrons in/out
What are the two main charge storage mechanisms in supercapacitors?
Electric double-layer capacitance (EDLC)
Pseudocapacitance
Explain the mechanism of Electric double-layer capacitance (EDLC)
When voltage is applied across the supercapacitor, ions in the electrolyte migrate toward the oppositely charged electrode surfaces.
Charges are accumulated at the electrode/electrolyte interface without any chemical change.
This forms an "electric double-layer," which is essentially two layers of charge separated by a very small distance, acting as a capacitor.
Energy is stored in this electric double layer (EDL) which is formed at the interface between the electrode surface and the electrolyte.
They store energy purely through electrostatic attraction.
Both electrodes are made from high surface area carbon materials, and energy storage occurs only at the electrode-electrolyte interface.
Explain the mechanism of Pseudocapacitance and Compare with EDLC
Charges are accumulated due to fast, reversible redox reactions at the electrode surface.
Materials like MnO2, ruthenium oxide or conducting polymers are used as electrodes.
These reactions contribute to more charge storage.
Hence, these devices have higher capacitance, higher energy density than EDLCs but typically lower power density.
Why are supercapacitors important?
They give high power density. Power density is the measure of how fast the device can deliver charge.
They possess long cycle life. Cycle life refers to the number of times the device can be subjected to charging-discharging and
In supercapacitors, energy storage occurs only at the surface of the electrode materials, hence they exhibit rapid charge-discharge capabilities but with lower energy density compared to batteries.
These characteristics make supercapacitors ideal for applications requiring quick bursts of energy or frequent charging cycles.
What electrode properties must be optimized to maximize the capacitance of a supercapacitor?
To obtain maximize capacitance of a supercapacitor, surface area of electrode materials should be maximum and distance between charge separation should be minimum according to C= εA/d where ε is the permittivity of the material.
What are Ultra-small asymmetric supercapacitors?
Ultra-small asymmetric supercapacitors are tiny devices designed for low-power, miniaturized electronics such as IoT nodes and wearables.
How does asymmetric supercapacitor store charge?
An asymmetric supercapacitor, store charge by combination of two different mechanisms.
One of the electrode stores charges by EDLC and other electrode stores charges by pseudocapacitance.
Two different electrode materials are used for the positive and negative terminals.
This design allows the supercapacitor to operate at a wider voltage range.
What is the relation between voltage and amount of energy stored in capacitor?
Energy stored in a supercapacitor is given by, E = ½ CV2 where E is energy, C is capacitance, and V is voltage.
Therefore, higher the operating voltage more is the amount of energy stored in a supercapacitor.
Explain Construction of Ultra-small asymmetric supercapacitor
Electrodes
In a small asymmetric supercapacitor, electrodes are small in size, they must exhibit high surface area to maximize the electrode-electrolyte interface in order to store more charges.
For this purpose, three-dimensional, nanostructured, porous materials are used as electrodes.
Cathode: This is the Pseudocapacitive electrode. Materials like MnO2, RuO2 or conducting polymers (PEDOT, polyaniline) are used as electrodes.
Anode: This is the EDLC electrode. It is made of a high surface area and good electrical conductivity carbon material like activated carbon, carbon nanotubes (CNTs), or graphene.
Separator: It is a thin, porous polymer membrane placed between the two electrodes. It is soaked in the electrolyte and allows ions to pass through while preventing the electrodes from touching and causing a short circuit.
Electrolyte: This is a solution containing positive and negative ions that can move freely. It can be an aqueous solution like potassium hydroxide (KOH). In flexible devices, organic electrolytes, ionic liquids, or polymer gel electrolytes are used.
Current collectors: Thin, flexible, conductive metal foils (like aluminum or copper) in contact with electrodes are used as current collectors. They receive or supply electrons to the electrodes
In ultra-small devices, all layers are sandwiched together to make device very thin
Explain Working of Ultra-small asymmetric supercapacitor
During Discharging, say when the supercapacitor is connected to a load (like an LED), the stored energy is released.
Electrons move thorough the external circuit from anode to cathode. This flow of electrons through the external circuit constitutes the electric current that powers the device. K+ ions move through the separator from anode to cathode
During Charging, a voltage is applied across the supercapacitor
How are Ultra-small supercapacitors applied in IoT?
IoT sensors typically require quick power bursts for wireless data transmission. Supercapacitors can be quickly charged and provide power bursts for wireless transmission
Energy harvesting systems commonly used in IoT devices like solar panels during daylight, vibration harvesters during movement, or thermal generators during temperature fluctuations generate power irregularly. Supercapacitors can be used to capture and store the energy harvested by other devices, then deliver it rapidly when needed for sensor operation and data transmission.
Supercapacitors exhibits long cycle life of over 1 million charge-discharge cycles. In IoT applications such as sensors embedded in infrastructure or remote monitoring systems, battery replacement would be impractical or impossible. Supercapacitors are ideal for such applications due to their long cycle life.
How are Ultra-small supercapacitors applied in Wearable Electronics?
Energy storage devices used in wearable devices must be lightweight, flexible, safe for body contact, and capable of handling frequent charging cycles.
Ultra-small supercapacitors can be charged rapidly. Full charge can be achieved in seconds to minutes, compared to hours for batteries. They can deliver high power density. Hence, in fitness trackers and smartwatches, supercapacitors can handle the power demands of periodic heart rate monitoring, GPS tracking, and wireless connectivity.
Supercapacitors are flexible and can be integrated into clothing or accessories. This can be used to power embedded sensors for health monitoring, activity tracking, or environmental sensing.
Differentiate between Batteries and Supercapacitors (Storage Technologies)
Batteries
Rely on chemical reactions
Ion transfer process between anode (+) and cathode (-) causes battery to heat up, expand and contract.
Loses Voltage Capacity → Short lifespan (even when not in use)
Battery’s ability to hold a charge slowly diminishes
High Specific energy or Energy Density → Best for long-term energy storage
Supercapacitors
Electrostatic
Negative and Positive charges are sepearated by Dielectric (insulator) sandwiched between plates → stores energy and releases in timely manner
Can keep same voltage rating for 20 yrs
High Power throughout
Low Specific Energy or Energy Density
Effective for brief surges of power
So, with batteries, while they can store a lot of energy and provide that energy over a long period, each charge and discharge cycle causes some degradation. Over time, the battery’s voltage and capacity gradually decline, meaning that the efficiency and the overall performance decrease with each cycle.
On the other hand, supercapacitors, even though they can’t hold as much energy for as long, maintain a consistent voltage and performance throughout their lifespan. This means that each time you use a supercapacitor, it’s just as effective and efficient as the first time, and it doesn’t suffer from the same gradual loss of capacity.
What are the types of Solar Energy utilization?
Direct solar power
Sunlight hits photovoltaic cell generating electricity.
Sunlight hits the dark absorber surface of solar thermal collector and warm the surface.
This heat energy is carried by a fluid circuit.
Indirect solar power
Photosynthesis
Photocatalysis
What is a Photovoltaic cell?
Solar photovoltaic cell is a device used to convert solar light directly into an electric current.
What is the Principle of Photovoltaic Cell?
PV cells rely on the photoelectric effect i. e., ability of certain elements to emit electrons when electromagnetic radiation of sufficient energy falls on it.
The photons possess certain amount of energy as evident from Planck quantum equation.
𝐸 = ℎ 𝑐 /𝜆 where, h is Planck’s constant, c is velocity of light and λ is the wavelength of the radiation.
Explain Construction of Photovoltaic Cell
A typical silicon solar cell consists of n-type material (phosphorus doped silicon) and a p-type (boron doped silicon) material made of silicon.
In the fabrication of device, these n-type and p-type materials are joined to form a p-n junction.
On top of n- type connecting front electrodes are placed. The metal back contact is attached to p-type layer.
An antireflective coating is filled in between the front electrodes to avoid the reflection of sunlight.
Finally p-type and n-type layers are joined externally to the circuit.
The complete solar cells are sealed to avoid the environmental contact.
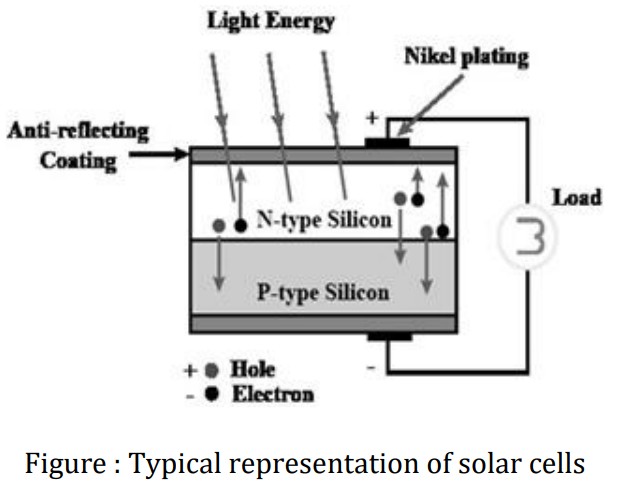
Explain Working of Photovoltaic Cell
When sunlight is incident on solar cells photon strikes on p-n junction.
Then electron hole pair will be created at the junction by the absorption of photon.
This electron hole pair diffuses towards respective layer based on affinity.
The electrons diffuse towards n-type layer and holes are diffused towards p-type layer.
These layers are connected externally through a circuit, hence current will be generated.
What are the Advantages of Solar Cells?
Solar cell is renewable energy which can be continuously drawn from the sun.
It is economic friendly energy because once it installed there will be minimum maintenance charges for small usage.
Solar energy is environment friendly and green energy because it doesn’t produce any greenhouse gasses and no pollutants.
It doesn’t involve any combustion reaction or radioactive residue hence no pollution.
What are the Disadvantages of Solar Cells?
Space utilization: Solar cells required large area for installation.
It requires high investment cost for a big power plant.
During the rainy season power production is less hence grid maintenance will be difficult.
Solar energy is produced only in the day time hence storage will be a big challenge.
Why are batteries unsuitable for powering modern IoT devices such as micro-sensors and medical implants?
Need replacement or recharging → impractical for:
Devices inside walls/bridges
Implants inside the human body
Bulky compared to micro-sensors
Contain harmful chemicals → environmental issues
What are Micro-Electro-Mechanical Systems?
MEMS stands for Micro-Electro-Mechanical Systems. Micro means they are very small systems, measured in micrometers. Electro-Mechanical means, they are a combination of tiny electronic circuits and tiny moving mechanical parts like gears, springs, and levers.
Energy Harvester is a device that "harvests" wasted energy from our surroundings and converts it into useful electricity. Just like a solar panel harvests sunlight, these devices harvest other forms of energy.
MEMS Energy Harvester is a microscopic machine designed to capture tiny amounts of wasted energy from the environment (like vibrations, heat, or movement) and turn it into electrical power for other small devices.
What is the Principle of MEMS?
MEMS Energy Harvester can harvest waste energy from the surroundings. Then, convert this ambient energy into electricity. This conversion process is called transduction. There are three main mechanisms through which energy is harvested by MEMS harvesters.
Piezoelectric Effect
Electromagnetic Effect
Electrostatic Effect
Explain Mechanism of Piezoelectric Effect in MEMS
Certain materials, called piezoelectric crystals like quartz, ZnO, PZT - lead zirconate titanate) exhibit Piezoelectric Effect.
When you mechanically stress them like squeeze, bend, or press them, their internal positive and negative charges get separated.
This separation of charge creates a voltage across the material. They generate electricity.
There are several sources of mechanical vibrations like machines vibrating, footsteps, moving vehicles, blood flow, etc.
Energy of these vibrations can be converted to electrical energy by attaching a MEMS device to these vibrations.
MEMS device has a tiny beam or strip called a cantilever. On top of this beam, a thin layer of piezoelectric material is deposited.
When the whole device vibrates, the mass makes the beam bend back and forth rapidly.
Bending causes mechanical strain in the piezoelectric layer.
Due to the piezoelectric effect, this strain produces electrical charges across the surfaces of the material.
Electrodes are attached to the piezoelectric layer to collect the charges. These charges create a voltage.
The tiny voltage is rectified and converted to DC using circuits and stored in a capacitor or battery.
This powers low energy electronics like MEMS sensors, wireless transmitters, or medical implants.
Explain Mechanism of Electrostatic Effect in MEMS
This method uses a capacitor which stores charge.
Ability of capacitor to store charge (capacitance, C) depends on the area of its plates (A) and the distance between them (d) According to the equation: C=εA/d
A MEMS harvester has a tiny capacitor with two plates. One plate is fixed, and the other is part of a movable mass-spring system.
When vibrations shake the device, the distance (d) between the plates changes, or the overlapping area (A) changes. This changes the capacitance (C).
A small initial voltage is needed to charge the capacitor first.
If the charge (Q) is kept constant, then when capacitance decreases due to vibration, the voltage (V = Q/C) increases.
This rising voltage is harvested through circuits generating a current. This cycle repeats with each vibration.
Explain Mechanism of Electromagnetic Effect in MEMS
This device is based on Faraday's Law of Electromagnetic Induction.
This law states that if you move a magnet near a coil of wire, a voltage is induced in the coil.
In a MEMS energy harvester, a tiny permanent magnet is attached to a vibrating cantilever, and a microcoil is fabricated on the fixed part of the chip.
When environmental vibration shakes the device, the magnet moves back and forth relative to the coil.
This changing magnetic field induces a current in the coil.
Piezoelectric method needs special material, electrostatic method needs pre-charging but electromagnetic harvesters are self-powered.
They can generate more power. But it is difficult to make really small, miniaturized compared to the other two.
List some Applications of MEMS
They are used in structural health monitoring. Sensors placed on a bridge or in a building can be powered by the vibrations from traffic or wind and continuously send data about the bridge's health, warning engineers of any damage.
They are used in medical implants like pacemakers. Current is produced from the vibrations from a person's heartbeat or body movement. This would eliminate the need for surgeries just to replace batteries.
They are used in wearable devices like smartwatches, fitness trackers, and even smart clothing. They get charged as you walk or run.
They are used in Wireless Sensor Networks. In agriculture, tiny sensors powered by wind could monitor soil moisture. In a factory, sensors powered by machine vibrations could monitor temperature and performance.
They are used in Aerospace. Sensors on an aircraft's wing can be powered using the vibrations from the flight itself.