chemistry - separate chemistry: transition metals, alloys & corrosion (5.1 - 5.7)
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
5.1 most metals are what kind of metals?
transition metals
5.1 typical properties of transition metals
high melting point
high density
form coloured compounds
catalytic activity of metals & their compounds (e.g. by iron)
5.2 what does oxidation of metals result in?
corrosion
rusting definition
corrosion of iron (or steel = mostly iron)
what is needed for rusting?
iron rusts when reacts with oxygen & water
5.3 preventing rusting of iron - exclusion of oxygen
keep air away
store metal in unreactive atmosphere of nitrogen/argon
paint metal
coat metal with plastic
oil metal
grease metal
5.3 preventing rusting of iron - exclusion of water
keep water away
use desiccant powder - absorbs water vapour
paint metal
coat metal with plastic
oil metal
grease metal
5.3 preventing rusting of iron - sacrificial protection
attach piece of magnesium/zinc to iron/steel object
magnesium & zinc oxidise more easily than iron (higher in reactivity series)
oxygen reacts with them not iron/steel object
protection continues until sacrificial metal corrodes away
5.4 electroplating definition
coat surface of one metal with thin layer of another metal
5.4 electroplating process - what you need
anode (+ electrode) - plating metal
electrolyte - solution containing ions of plating metal
cathode (- electrode) - metal object
5.4 electroplating process - what happens (e.g. electroplating copper ring with silver)
direct current flows through electrodes & electrolyte
silver anode (+): silver atoms —lose electrons→ silver ions in electrolyte
+ silver ions in electrolyte → - copper ring
+ silver ions —gain electrons→ deposited as silver atoms
current flows for longer = silver layer on ring becomes thicker
5.4 electroplating - improve appearance of metal objects
silver & gold: attractive transition metals; expensive
silver/gold electroplated onto cheaper ‘base metals’ (e.g. copper/nickel)
produces jewellery: attractive; cheaper than solid silver/gold
5.4 electroplating - improve resistance to corrosion of metal objects (e.g. chromium)
chromium: transition metal; resists corrosion
steel objects (e.g. vehicle parts) ‘chrome plated’ by electroplating
thin chromium layer stops air & water reaching steel - prevents rusting
5.4 electroplating - improve resistance to corrosion of metal objects (e.g. zinc)
iron/steel objects coated with zinc - galvanising
thin zinc layer stops water reaching iron/steel & acts as sacrificial metal - improves corrosion resistance
5.4 electroplating - improve resistance to corrosion of metal objects (e.g. tin)
food cans made from steel
inner surface protected from rusting - electroplating with tin
tin: doesn’t react with air/water (at room temp.)
tin layer stops air & water reaching steel
tin layer damaged = steel rusts faster - iron more reactive than tin, acts as sacrificial metal to protect tin
alloy definition
mixture of metal element with one/more elements (usually metals)
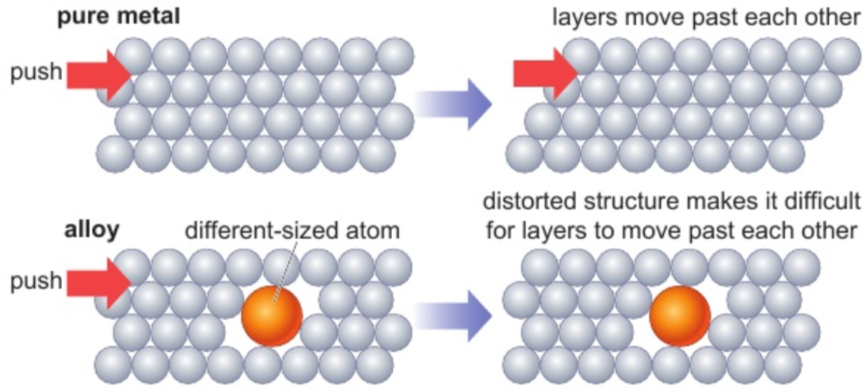
5.5 why does converting pure metals into alloys increase strength of product? (using models)
solid pure metal:
atoms all same size & regularly arranged in layers
layers move past each other if enough force applied (metals = malleable & ductile)
alloy:
atoms of other elements present may be diff. sizes
distort regular structure
more difficult for layers to slide past each other
alloys often stronger than pure metals they contain (even though usually still malleable & ductile)
5.6 why is iron alloyed with other metals to produce alloy steels?
alloy steels made by deliberately adding other elements to iron
can create large range of alloy steels with diff. properties by varying their composition
e.g. stainless steels:
contain chromium - reacts with oxygen in air
layer of chromium oxide forms
thick enough to stop air & water reaching metal
thin enough to be transparent
metal scratched - more chromium reacts to replace layer
what do uses of metal/alloy depend on?
chemical properties - e.g. resistance to corrosion
physical properties - e.g. density, ability to conduct electricity
5.7 how are uses of metals related to their properties (& vice versa) - aluminium vs copper
both:
resist corrosion
aluminium:
does not conduct electricity as well as copper
stronger, cheaper & less dense than copper - used for overhead electrical cables
5.7 how are uses of metals related to their properties (& vice versa) - copper vs gold
both:
resist corrosion
malleable
ductile
good conductors of electricity - can be used for electrical wiring
copper:
cheaper than gold - used for most electrical wiring
gold:
expensive - used in tiny amounts in computers
5.7 how are uses of metals related to their properties (& vice versa) - aluminium vs magnalium
magnalium:
less dense, stronger & better resistance to corrosion than aluminium - used to make strong but lightweight metal parts
5.7 how are uses of metals related to their properties (& vice versa) - copper vs brass
both:
resist corrosion
copper:
better conductor of electricity than brass
brass (alloy of copper & zinc):
stronger than copper - used for making electrical plug pins