AP Biology Unit 3: Cellular Energetics
1/52
Earn XP
Description and Tags
AP Biology unit 3 cellular energetics study guide
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
Metabolism
Encompasses all the chemical reactions that occur within an organism.
Energy and Macromolecule Requirements
Organisms constantly require energy and macromolecules to sustain their metabolic processes
Catabolic Pathways
These pathways break down complex molecules into simpler components, releasing energy in the process.
Anabolic Pathways
Conversely, these pathways use energy to synthesize complex molecules from simpler ones.
Function as Catalysts
Enzymes are important as they act as catalysts in metabolic pathways, significantly speeding up chemical reactions without being consumed.
Example: The enzyme lactase catalyzes the hydrolysis of lactose into glucose and galactose.
Bond Dynamics
All chemical reactions involve the breaking and forming of bonds, processes that inherently require energy.
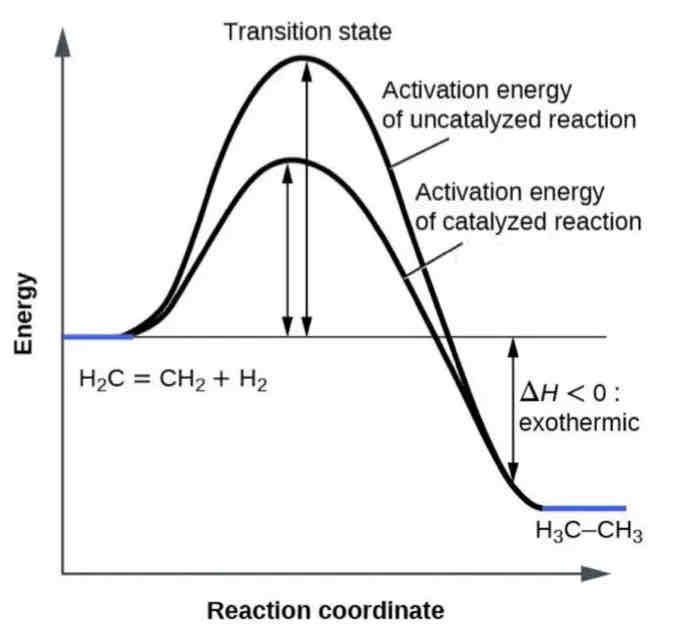
Activation Energy (EA)
Activation energy is the initial energy needed to start a chemical reaction-akin to pushing a ball uphill before it can roll down.
Enzyme Activity
Enzymes function by lowering the activation energy required for a reaction, making it easier for the reaction to occur at a faster rate.
Enzymatic Catalysis
Efficiency: By reducing the activation energy, enzymes allow reactions to proceed more rapidly and at lower energy costs.
Reaction Mechanism: Enzymes provide a platform where reactants can come together in an optimal orientation, which facilitates the formation of transition states and thereby lowers the activation energy needed.
Enzyme Specificity
Enzymes are highly specific catalysts, meaning they typically catalyze only one specific type of chemical reaction.
Example: Lactase is an enzyme that specifically catalyzes the breakdown of lactose into galactose and glucose.
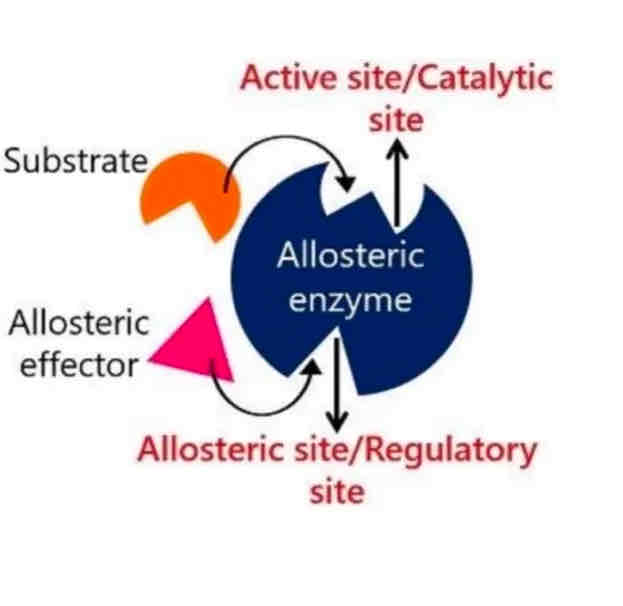
Enzyme and Substrate Interaction
Substrate: The substrate is the specific reactant that an enzyme acts upon. For instance, in the reaction catalyzed by lactase, lactose serves as the substrate.
Binding: Enzymes are proteins that bind to their substrate at a region known as the active site.
Mechanism of Enzymatic Action
Active Site Binding: Substrates enter the enzyme's active site and bind to it.
InducedFit Model: Upon substrate binding, the enzyme changes its shape slightly to better enfold and hold the substrate, a process known as "induced fit.”
Conversion to Products: While bound to the enzyme, the substrate is transformed into the products) of the reaction.
Release of Products: Once the conversion is complete, the products are released from the active site.
Reusability of the Enzyme: After the products are released, the active site becomes available again for new substrate molecules to bind and undergo the same reaction.
Reaction Sequence
This can be generalized as follows:
E + S → ES → EP→ E + P
E: Enzyme
S: Substrate
ES: Enzyme-substrate complex
EP: Enzyme-product complex
P: Product
Influence of Environmental Factors
Enzyme activity is influenced by several environmental conditions, including temperature, pH, substrate concentration, and the presence of inhibitors.
Temperature Effects
Reaction Rate: Increasing the temperature generally increases the rate of enzymatic reactions because substrates collide with active sites more frequently.
Denaturation: However, excessively high temperatures can disrupt the weak chemical bonds that maintain the protein's structure, leading to denaturation, where the enzyme loses its functional shape and becomes inactive. This process is sometimes reversible.
pH Effects
Optimal pH Range: Enzymes function within specific pH ranges.
Alterations from this range can also lead to denaturation, as excessive [H+] ions can alter the protein's shape through disruptions in hydrogen bonding.
Reaction Rate Increase
Higher substrate concentrations typically increase the reaction rate up to a point. This is because more molecules are available to collide with and react at the enzymes active sites.
Saturation Point
The reaction rate plateaus when all enzyme active sites are occupied, a state known as saturation.
Reaction Rate Limitation
An increase in enzyme concentration will also increase the reaction rate, provided there is enough substrate present to bind to the available active sites.
Competitive Inhibitors
These molecules compete with the substrate for binding at the enzyme's active site, effectively blocking the substrate from binding.
Noncompetitive Inhibitors
These bind to a different part of the enzyme, causing a change in the enzyme's shape that makes the active site less effective or unable to bind the substrate.
Allosteric Regulation
This form of regulation involves molecules binding to sites other than the active site (allosteric sites), which affects the enzyme's function by inducing changes in its conformation.
Energy Dynamics
Life processes require a continuous input of energy, where energy input generally exceeds energy output. This constant flow is essential for sustaining life.
Transformation and Conservation of Energy
In biological systems, energy is converted from one form to another but is never destroyed, adhering to the first law of thermodynamics, which states that energy cannot be created or destroyed.
Non-recyclability of Energy
Although materials like carbon can be recycled, energy itself, once used, is often released as heat and cannot be reused by the organism.
Thermodynamic Laws
Life processes do not violate the laws of thermodynamics; instead, they are exemplary models of these principles in action.
Exergonic Reactions
These reactions result in a net release of energy, making them spontaneous. The energy released from exergonic reactions is often harnessed to power other cellular processes.
Endergonic Reactions
Conversely, these reactions absorb free energy from their surroundings and thus require an input of energy to proceed, making them non-spontaneous.
Energy Coupling
This is the process by which the energy released from exergonic reactions is used to drive endergonic reactions, a critical aspect of metabolic pathways.
Controlled Energy Transfers
Cellular pathways are carefully sequenced to ensure that energy transfers are controlled and efficient.
The product of one reaction often serves as the reactant for the next, facilitating a continuous flow of energy through various biochemical pathways.
Photosynthesis
CO2 + H2O + Light → C6H1206 + 02
Light reactions and the Calvin cycle
Process | Light Reactions | Calvin Cycle |
Location | Thylakoid membranes | Stroma of the chloroplast |
Energy Source | Sunlight | ATP and NADPH produced in the light reactions |
Main Function | Convert light energy into chemical energy (ATP and NADPH) | Use ATP and NADPH to convert CC? into glucose |
Key Events | - Photon absorption by - Carbon fixation of chlorophyll<br>- Water splitting (oxygen evolution) - Electron transport chain creates a proton gradient for ATP synthesis | CO2 into organic molecules - Reduction and regeneration of the CO2 acceptor |
Output | ATP, NADPH, 02 | Glucose, ADP, NADP+ |
Photosystems in the Thylakoid Membrane
Composed of a reaction center complex surrounded by multiple light-harvesting complexes that contain chlorophyll.
Chlorophyll molecules absorb light, causing an electron to become
"excited."The excited electrons energy is transferred among molecules in a process where each recipient molecule is reduced as it gains electrons.
Photosystem II
Absorption of light excites an electron which is then captured
by an electron acceptor.Water is split to replace the lost electron, releasing hydrogen ions and oxygen.
Electron Transport Chain
Electrons move from Photosystem Il to Photosystem I, pumping hydrogen ions into the thylakoid and creating a proton gradient.
Photosystem I
Light re-excites the electron before it is transferred to another acceptor.
The electron is used, along with an enzyme, to reduce NADP+ into NADPH (a high-energy molecule).
ATP Synthesis
The proton gradient across the thylakoid membrane gives ATP to produce ATP.
ATP Synthase Function
Mechanism: ATP synthase is a crucial enzyme that harnesses the energy from a proton gradient across the thylakoid membrane to synthesize ATP. As protons flow back across the membrane through the ATP synthase channel, the enzyme catalyzes the addition of a phosphate group to ADP, forming ATP.
Reaction: ADP + P → ATP
Role in Photosynthesis: This process provides the energy currency for many cellular processes, including the Calvin cycle in photosynthesis.
The Calvin Cycle
The Calvin cycle, also known as the light-independent reactions or dark reactions, uses the ATP and NADPH produced during the light reactions to convert carbon dioxide into organic carbohydrates.
ATP
Supplies the energy needed for the biochemical reactions within the Calvin cycle.
NADPH
Provides the reducing power (electrons) necessary to convert the fixed carbon dioxide into a more reduced (energy-rich) form as part of carbohydrate molecules.
Carbon Fixation
CO2 molecules are attached to five-carbon sugars by the enzyme RuBisCO, creating unstable six-carbon compounds that immediately split into two three-carbon molecules.
Reduction Phase
ATP and NADPH are used to convert the three-carbon molecules into glyceraldehyde-3-phosphate
(G3P), a three-carbon sugar. Some G3P molecules exit the cycle to be used in other metabolic pathways, while others remain to be recycled.
Regeneration of the CO2 Acceptor
ATP is used to convert some of the G3P back into RuBP (ribulose-1,5-bisphosphate), the molecule that accepts CO2, allowing the cycle to continue.
Cellular Respiration
This is a catabolic process by which cells generate ATP through the breakdown of biological macromolecules. It is a fundamental energy-producing pathway in many organisms.
Aerobic Respiration
This form of respiration requires oxygen to convert organic fuel into ATP, carbon dioxide, and water.
Equation: C6H1206 + 602 → 6CO2 + 6H20 + ATP
Efficiency: Aerobic respiration is more efficient at generating ATP
compared to anaerobic processes.
Aerobic Respiration phases
Glycolysis: Occurs in the cytosol, breaking glucose into two pyruvate molecules, producing 2 ATP and 2 NADH.
Krebs Cycle (Citric Acid Cycle): Pyruvate is converted to acetyl CoA, which enters the Krebs cycle within the mitochondria, producing additional ATP, NADH, and FADH2.
Electron Transport Chain (ETC): Electrons from NADH and FADH2 are transferred through a series of proteins, generating a proton gradient that drives the synthesis of a large amount of ATP through oxidative phosphorylation.
Fermentation
This is an anaerobic process where organic fuels are degraded without the use of oxygen.
Characteristics: It is less efficient than aerobic respiration, generally utilized by most prokaryotes and some eukaryotes under anaerobic conditions.
Pathway: In fermentation, glycolysis is followed by the conversion of pyruvate into other molecules like lactate or ethanol, allowing NAD+ to be regenerated and glycolysis to continue producing a small amount of ATP.
Phosphorylation
This is the process of adding a phosphate group to ADP to form ATP, a key step in energy transfer within cells.
Phosphorylation types
Substrate-Level Phosphorylation: Direct transfer of a phosphate group to
ADP during glycolysis and the Krebs cycle.Oxidative Phosphorylation: Occurs in the mitochondria during the electron transport chain, where energy from electrons is used to pump protons across the mitochondrial membrane, creating a gradient used by ATP synthase to produce ATP.
Krebs/Citric Acid Cycle
The Krebs cycle takes place in the mitochondrial matrix, the innermost compartment of mitochondria.
This cycle is a key component of cellular respiration where pyruvate, derived from glucose during glycolysis, is fully oxidized to produce energy carriers.
CO2 Release: During the cycle, carbon dioxide is released as a byproduct from the decarboxylation of organic intermediates.
Energy Production: The cycle generates 1 ATP per turn through substrate-level phosphorylation.
It also produces 3 molecules of NADH and 1 molecule of
FADH2. These are energy-rich carriers that store potential energy to be used in the electron transport chain.
Mitochondrial Electron Transport Chain (ETC)
Location: The electron transport chain is located along the inner mitochondrial membrane, which provides the necessary surface for complex protein structures involved in electron transfer.
Function: Energy Release and Transfer: Electrons from NADH and FADH2 are transferred through a series of protein complexes and electron carriers within the inner mitochondrial membrane.
As electrons move through the ETC, the energy used is released to pump protons (H+) from the mitochondrial matrix into the intermembrane space, creating a proton gradient.
oxidative phosphorylation
ATP Synthesis: The proton gradient drives protons back into the matrix through ATP synthase, a process known as chemiosmosis.
This movement powers the synthesis of ATP.This phase of respiration is highly efficient, producing the majority of ATP during cellular respiration.