Chapter 2 chem ORGANIC CHEMISTRY
1/333
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
334 Terms
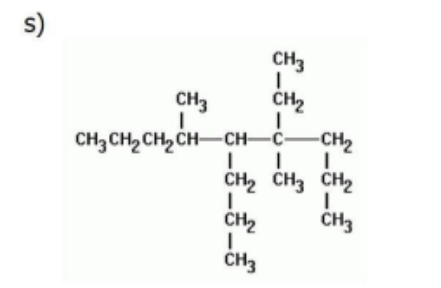
Name
4-ethyl-4,6-dimethyl-5-propylnonane

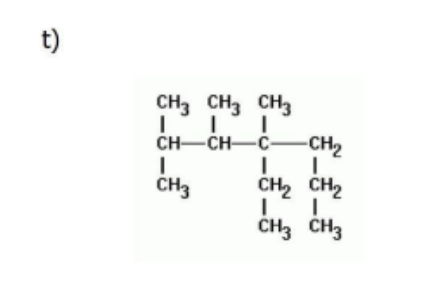
Name
4-ethyl-2,3,4-trimethylheptane

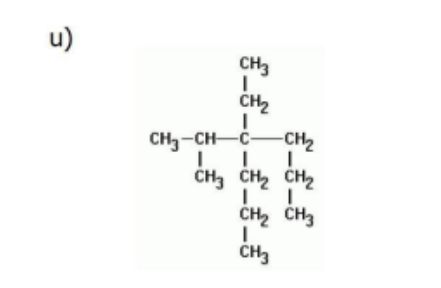
Name
4-ethyl-4-isopropylheptane
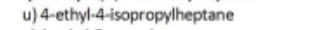
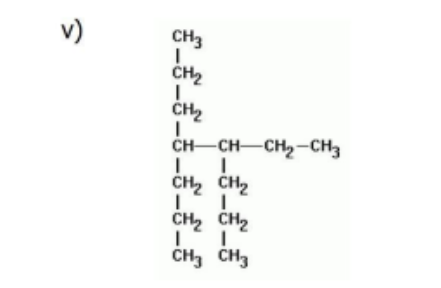
Name
4-ethyl-5-propyloctane
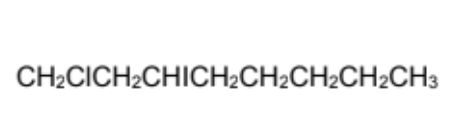
Name
1-chloro-3-iodooctane
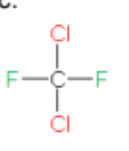
Name
dichlorodifluoromethane
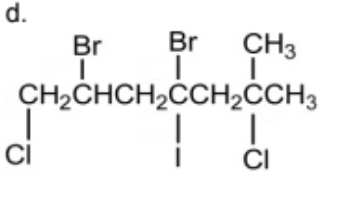
Name
2,4-dibromo-1,6-dichloro-4-iodo-6-methylheptane
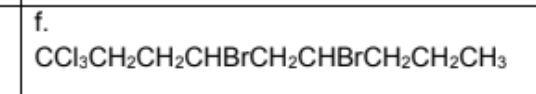
Name
4,6-dibromo-1,1,1-trichlorononane
General formula for alkanes?
CnH2n+2
General formula for cycloalkanes?
CnH2n
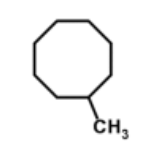
name
methylcyclooctane
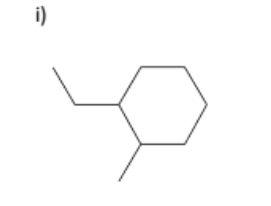
name
1-ethyl-2-methylcyclohexane
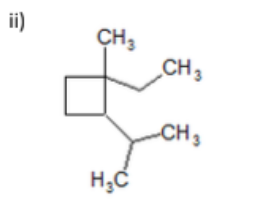
name
1-ethyl-2-isopropyl-1-methylcyclobutane
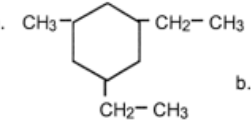
Name
1,3-diethyl-5-methylcyclohexane
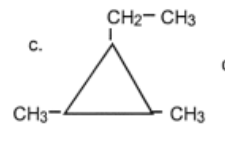
name
1-ethyl-2,3-dimethylcyclopropane
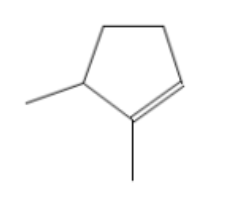
name
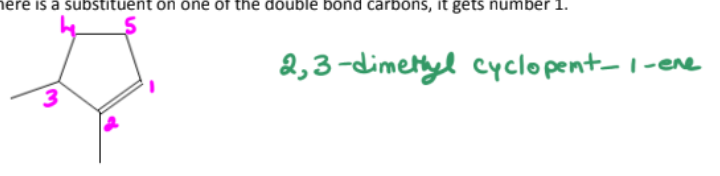
name
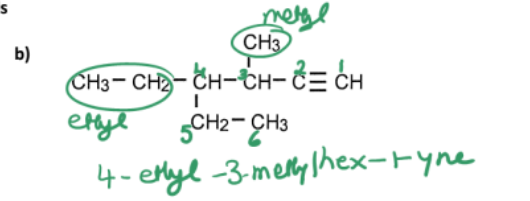
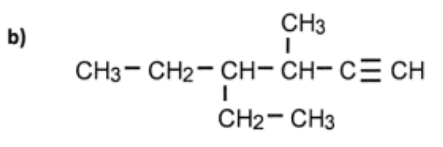
name
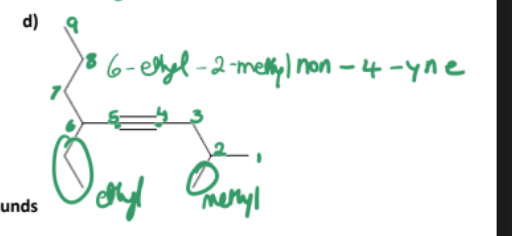
Draw:
3-ethyl-5,5-dimethylhept-2-ene
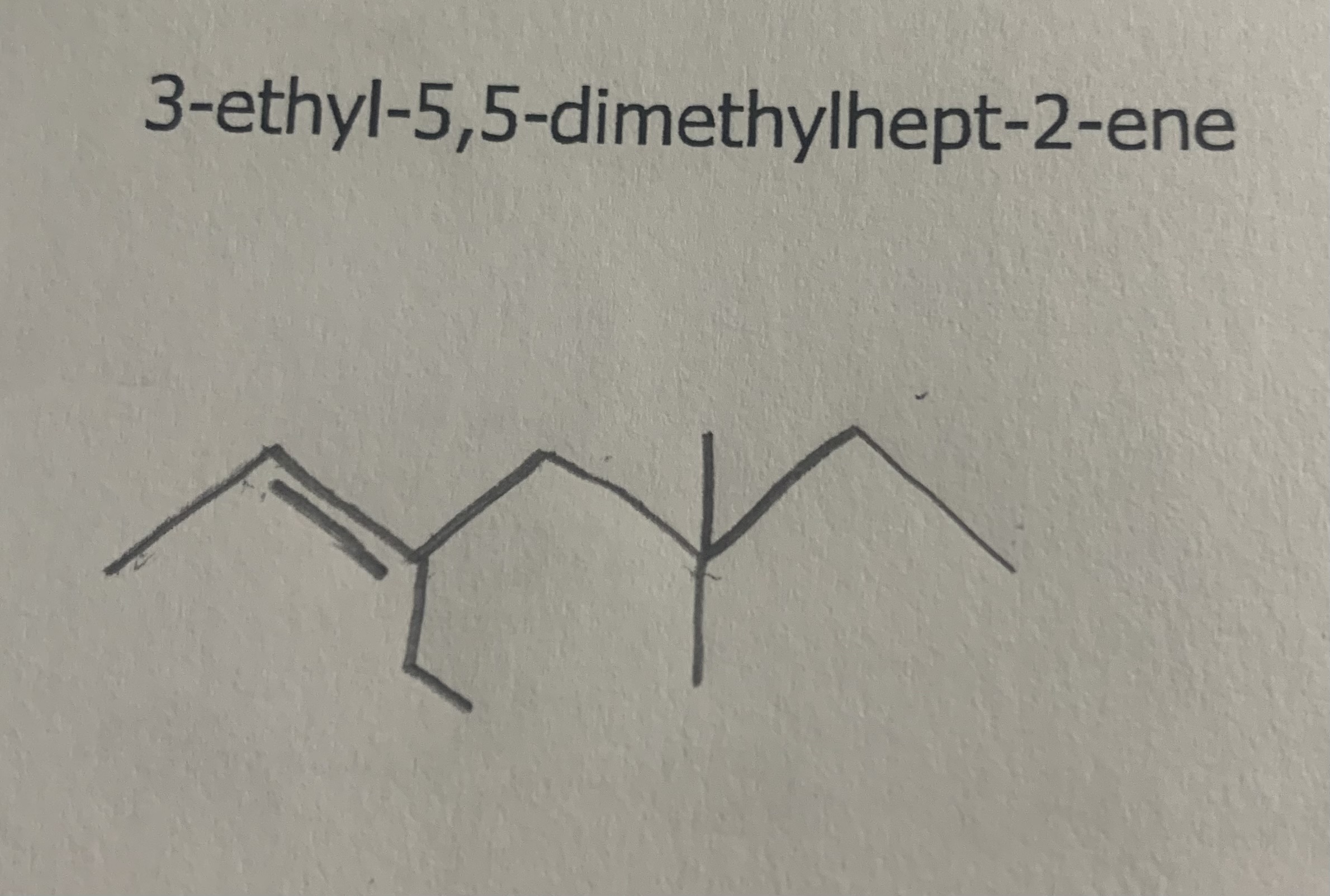
Draw:
3,4,5-trichloropenta-1,3-diene
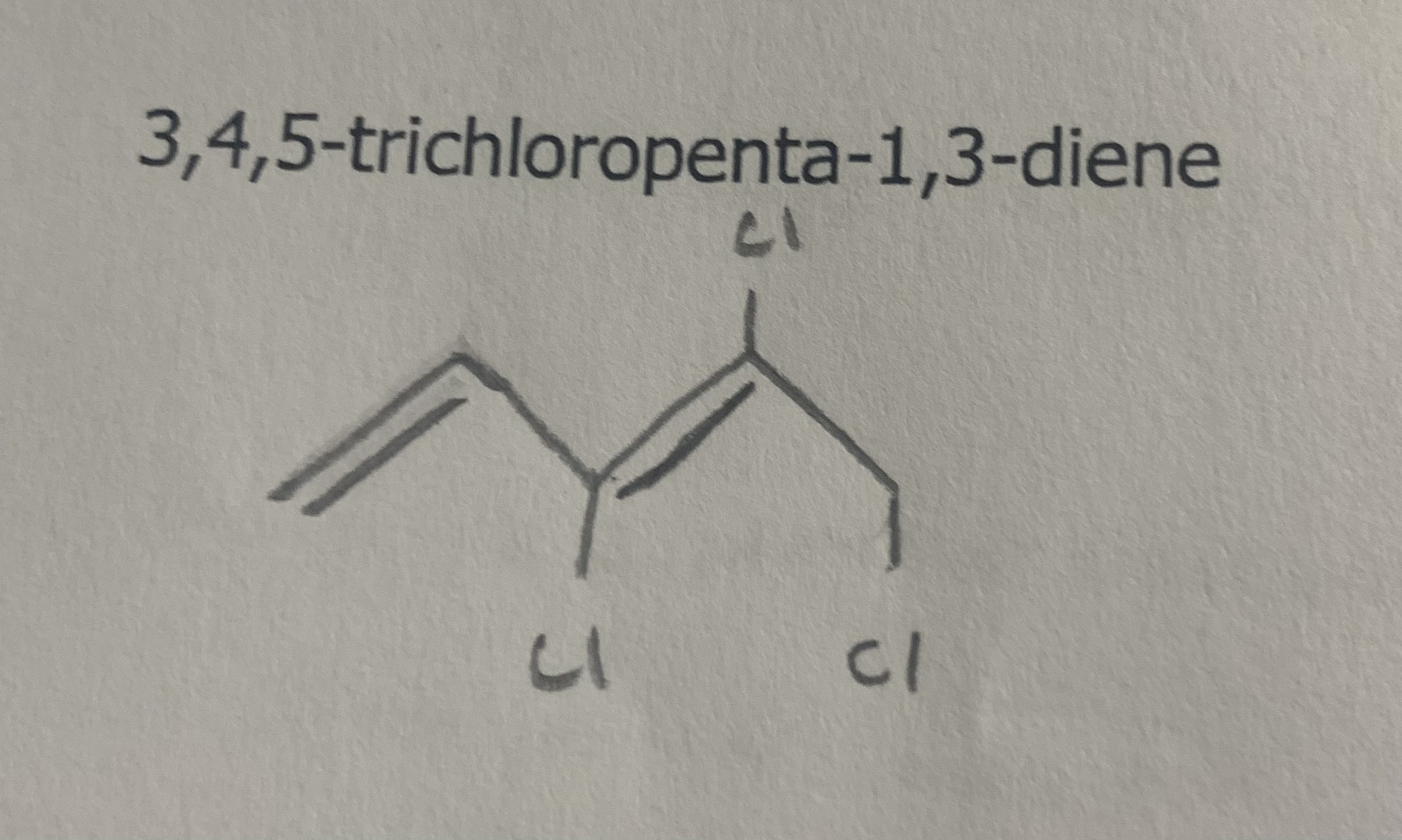
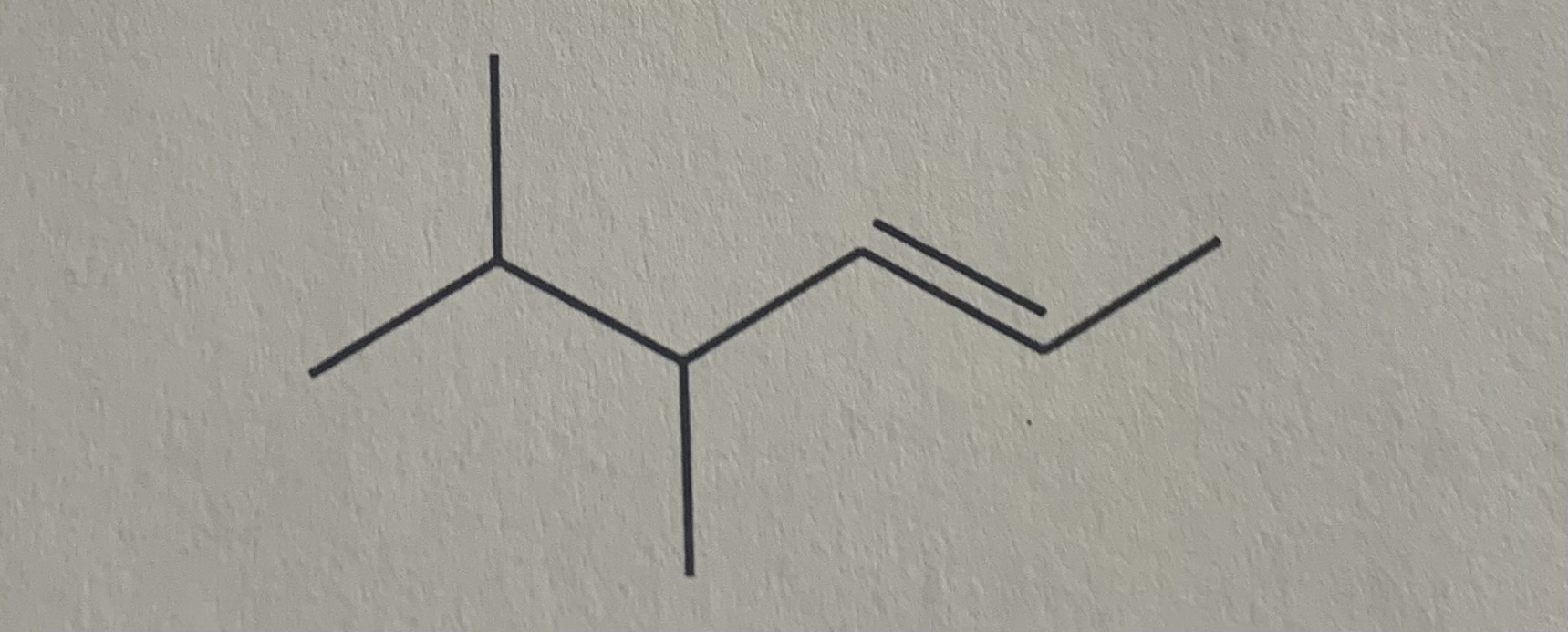
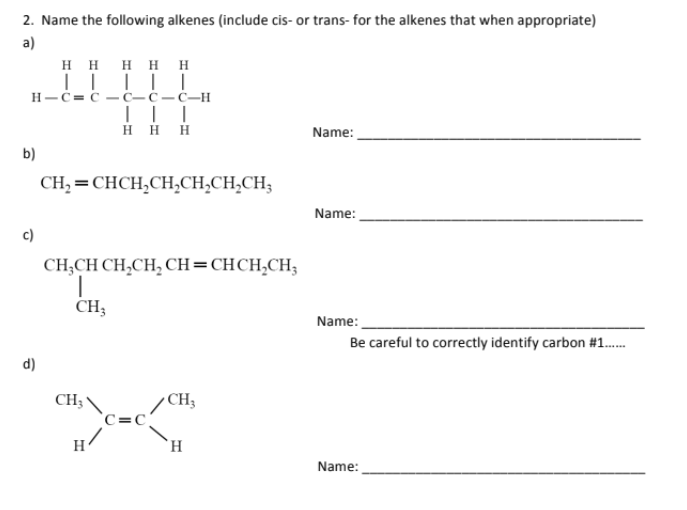
Name
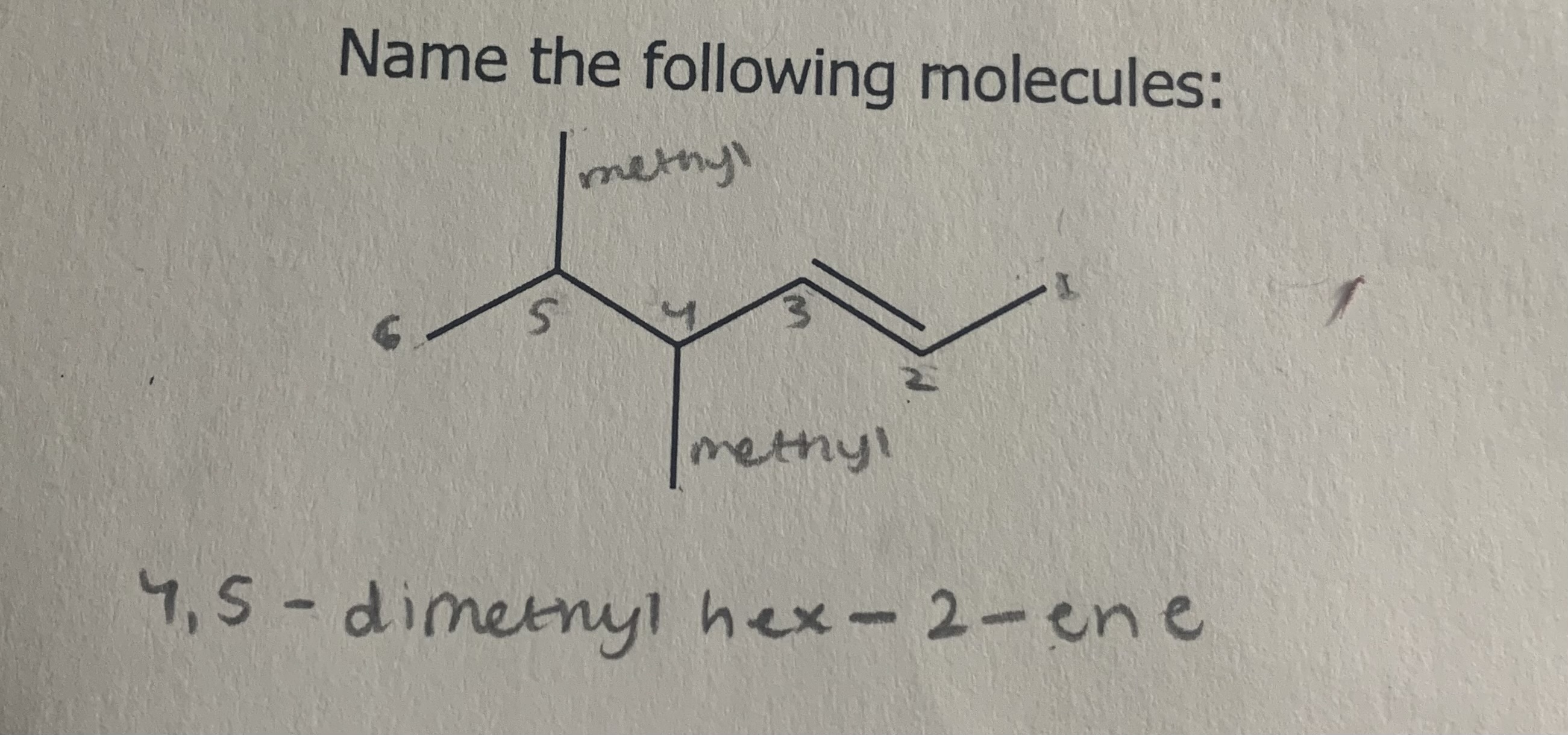
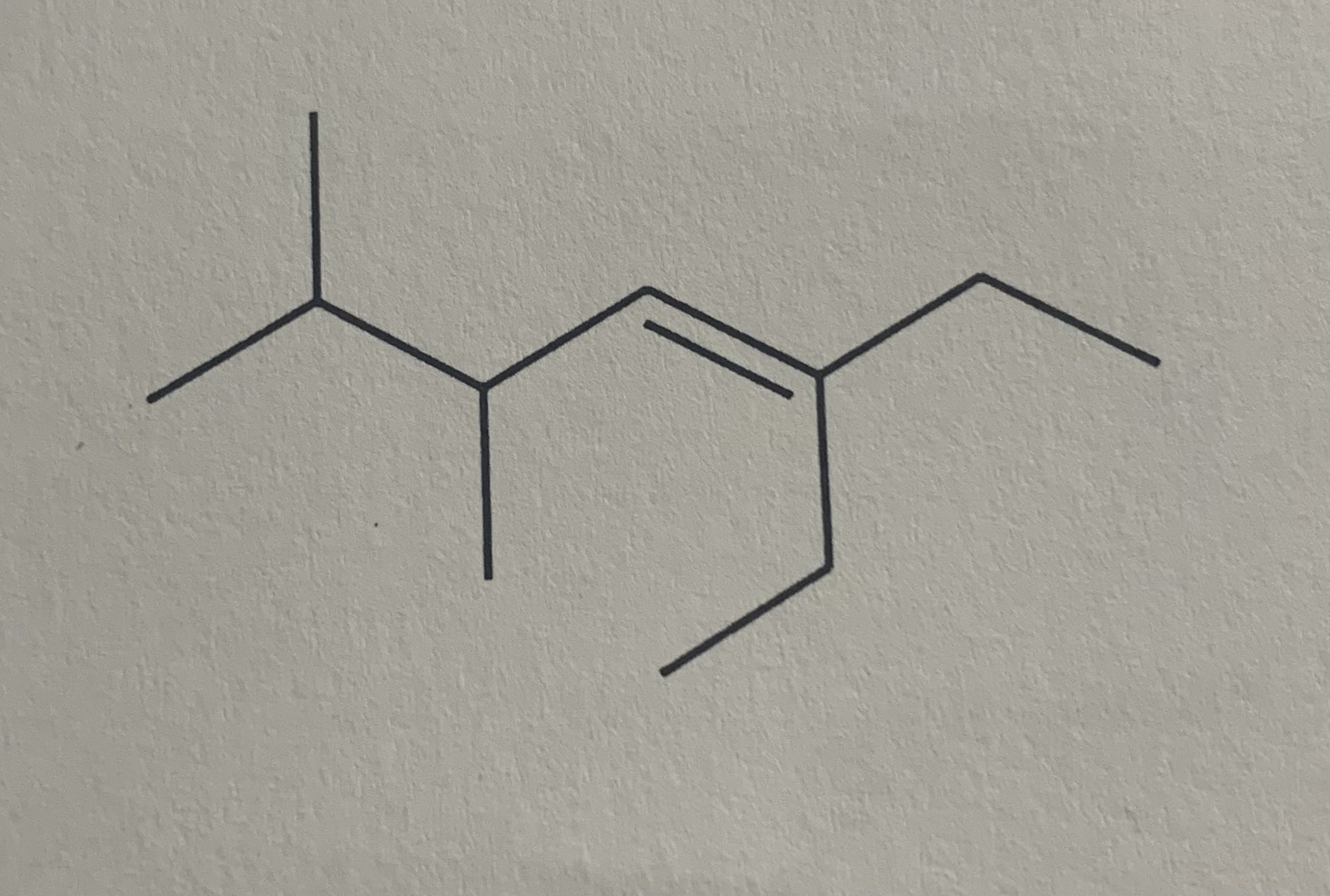
Name
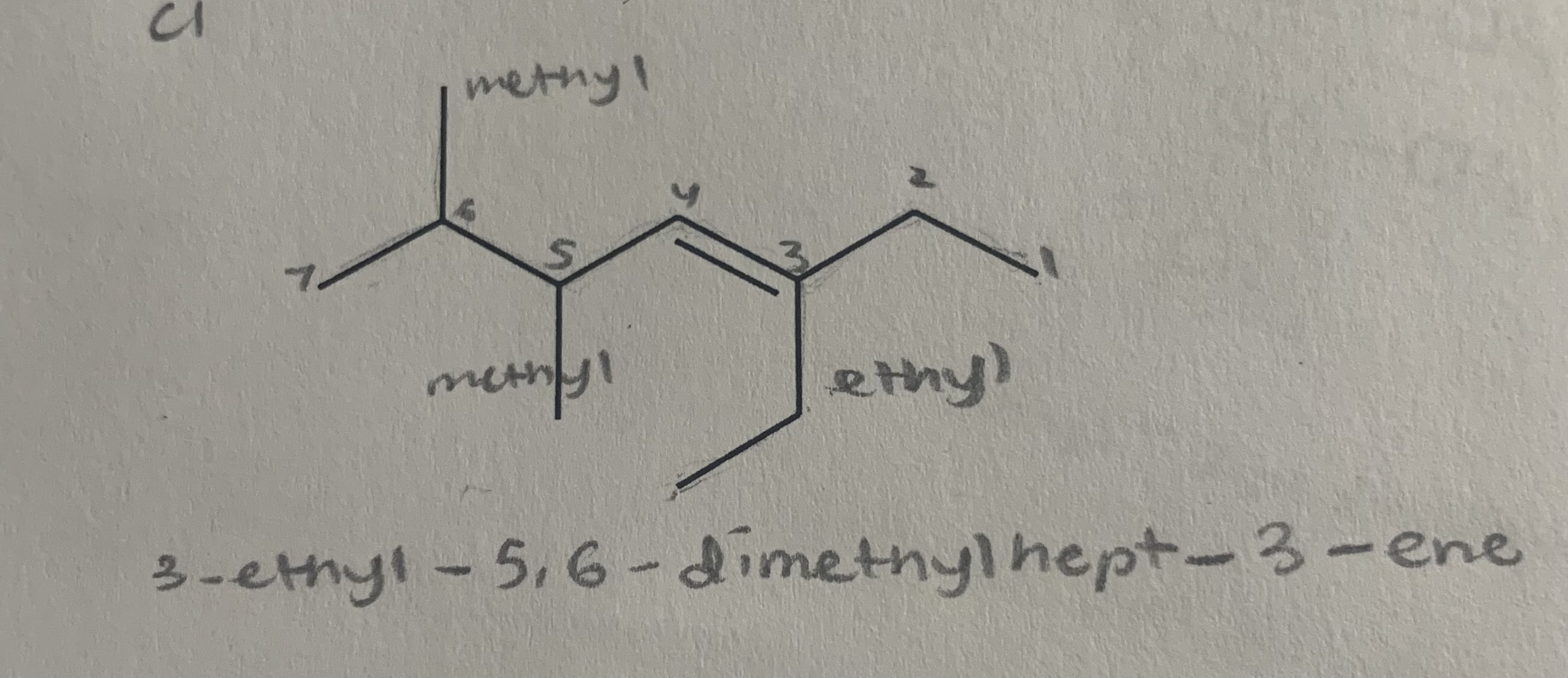
Name
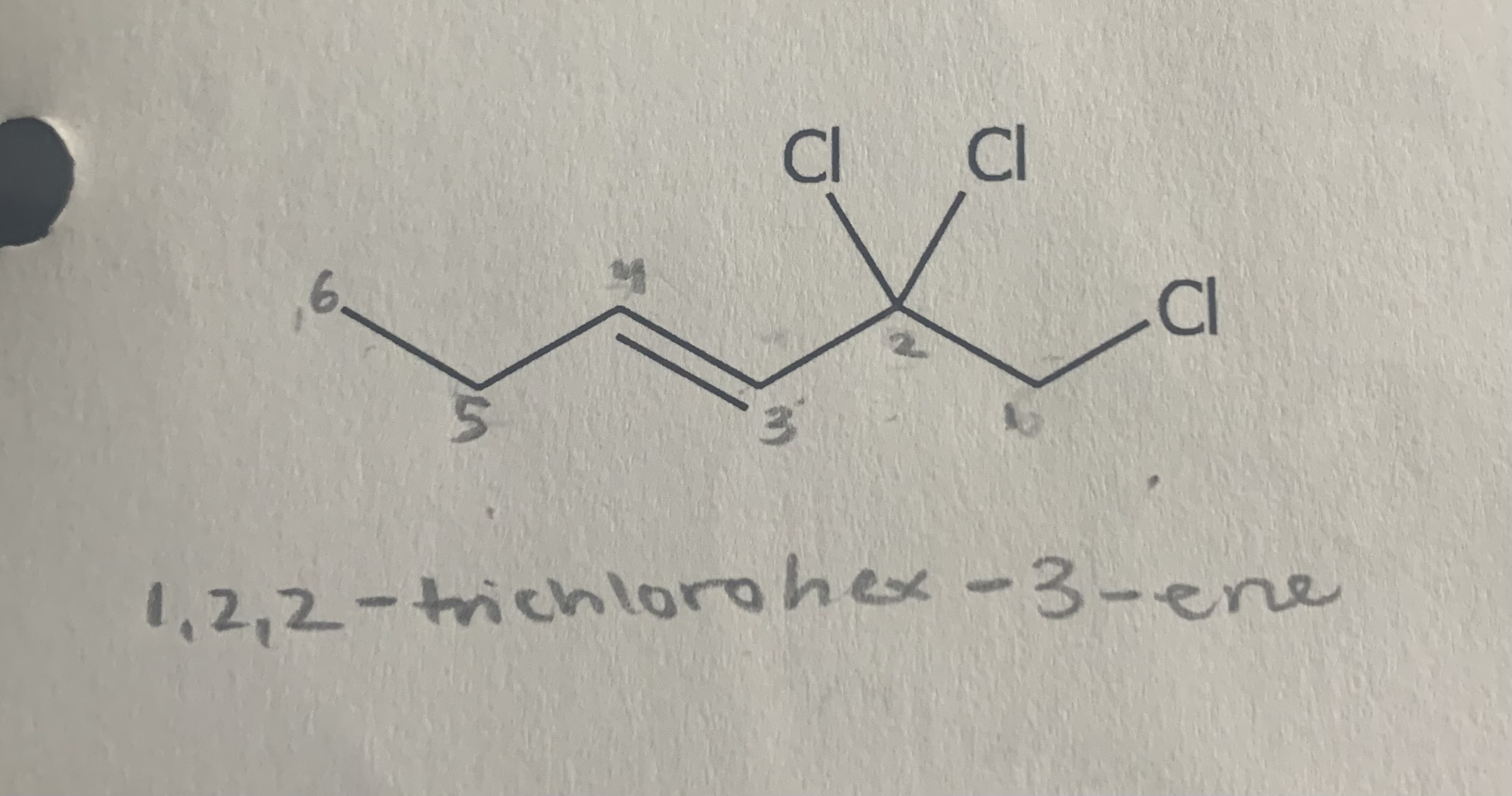
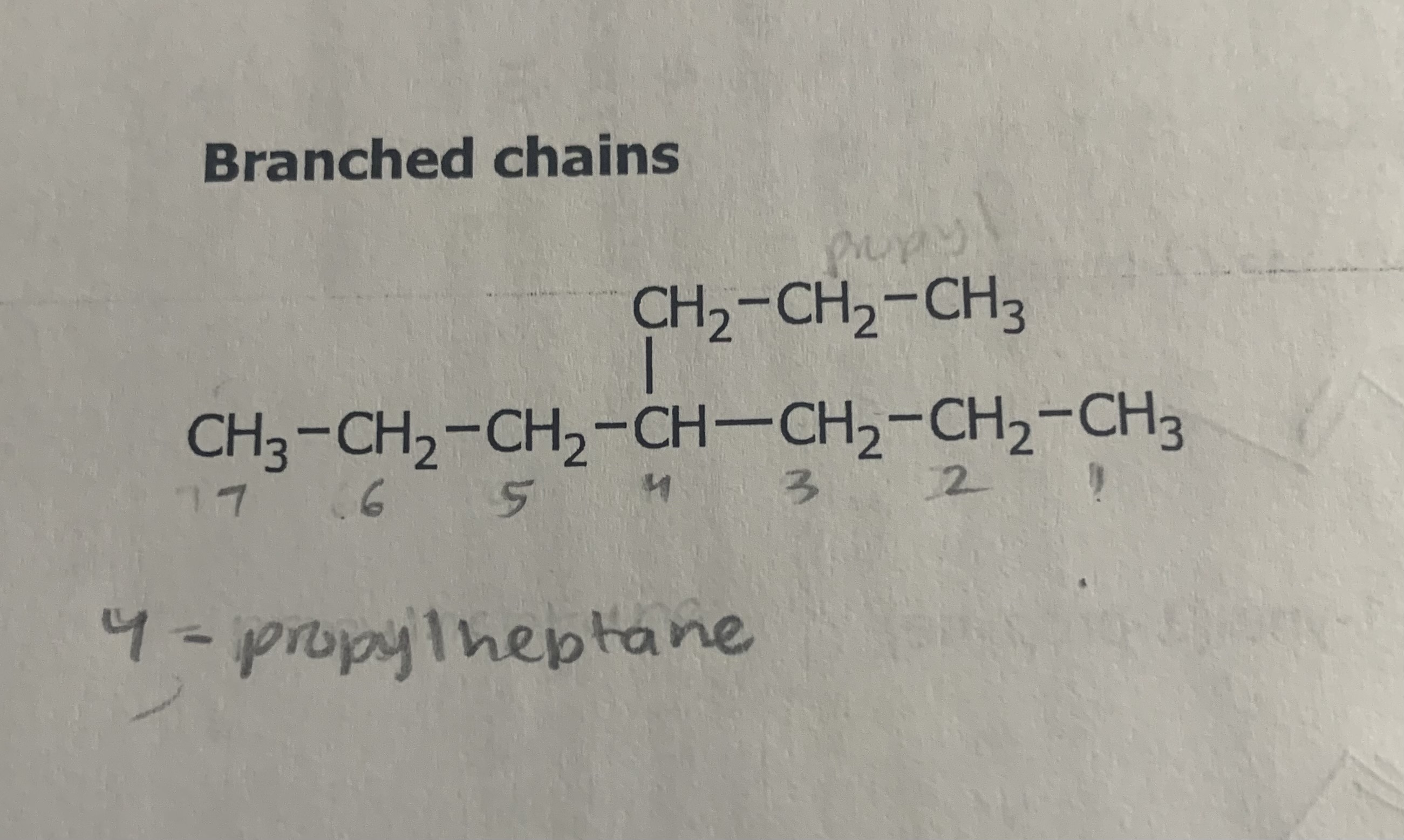
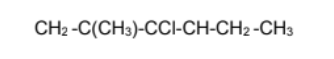
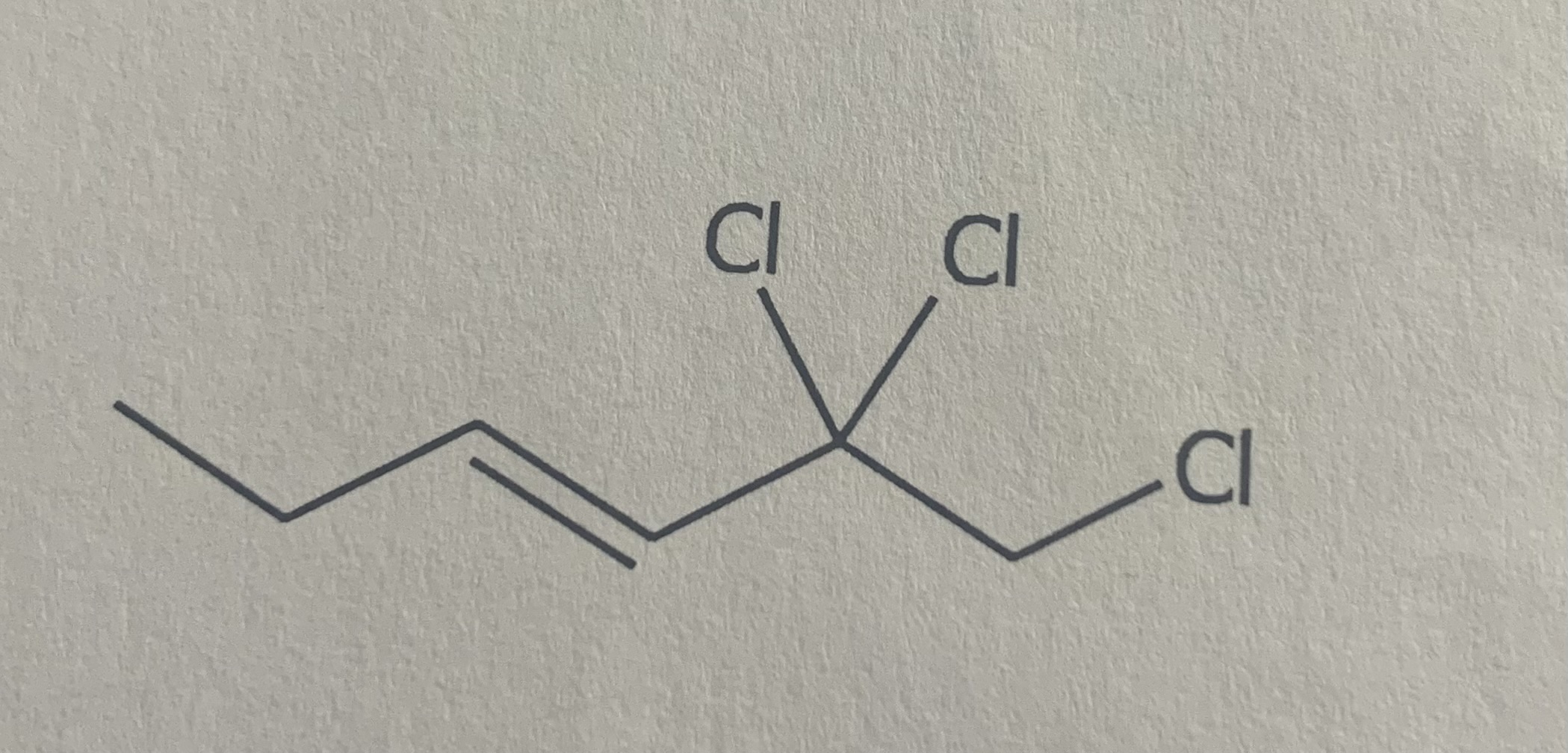
Name
3-chloro-2-methylhexane
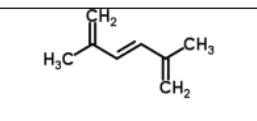
Name
2,5-methylhex-1,3,5-triene
draw:
3-isopropylhex-3-ene
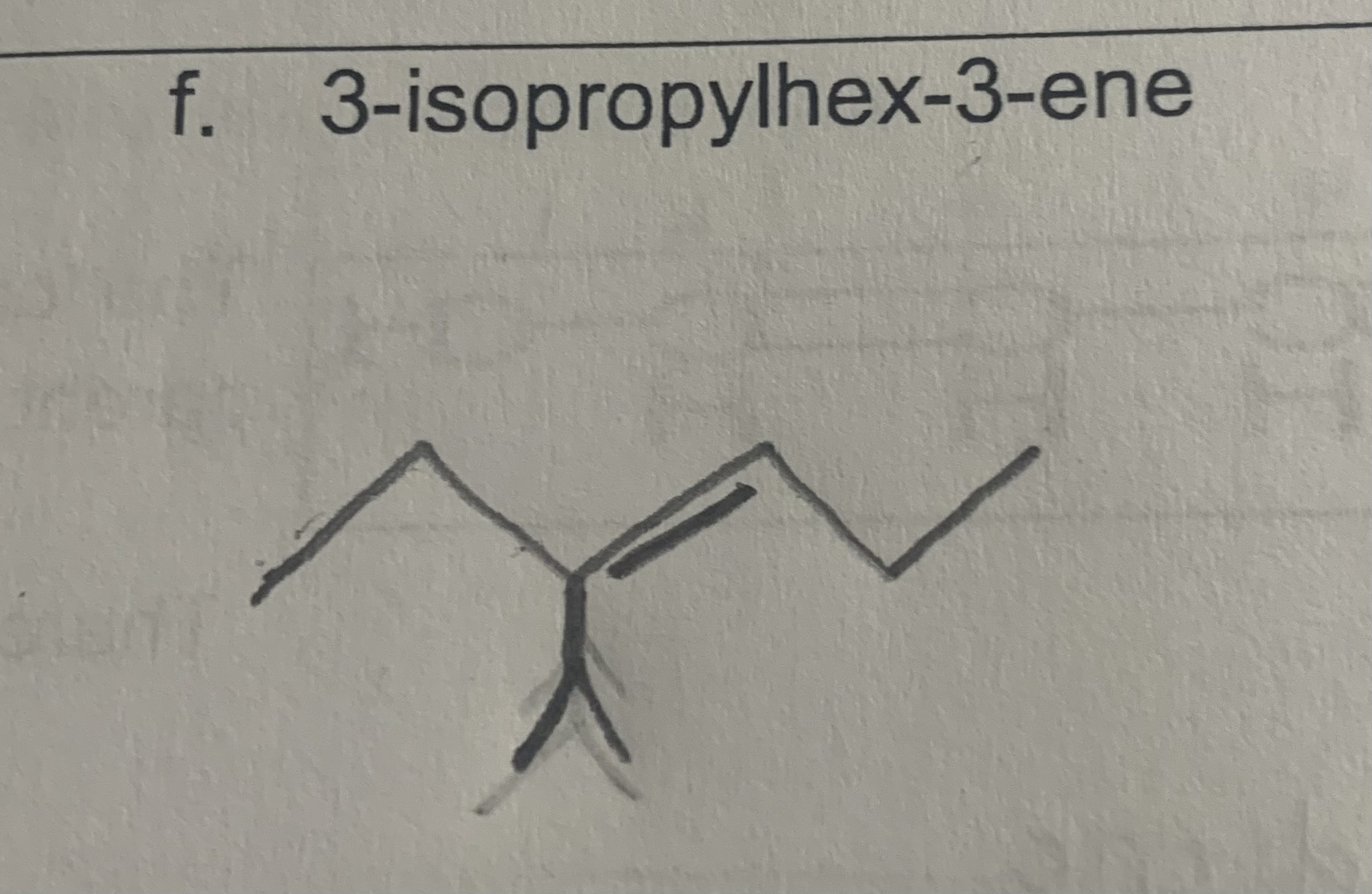
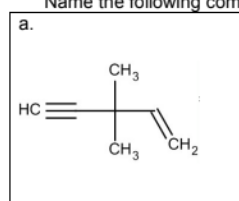
Name:
3,3-dimethylpent-1-en-5-yne
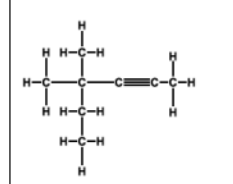
name
4,4-dimethylhex-2-yne
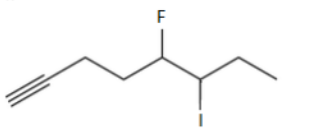
name
5-fluoro-6-iodooct-1-yne
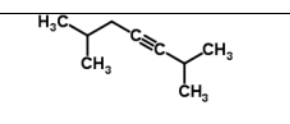
name
2,6-dimethylhept-3-yne
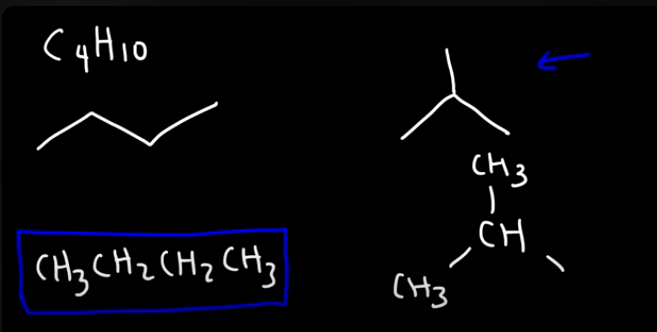
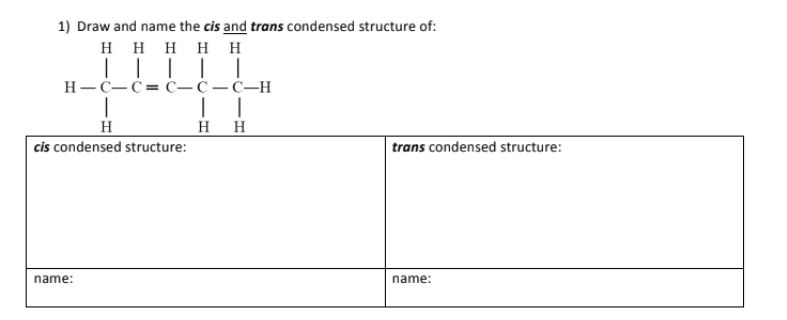
Draw all the constitutional isomers of:
C4H10
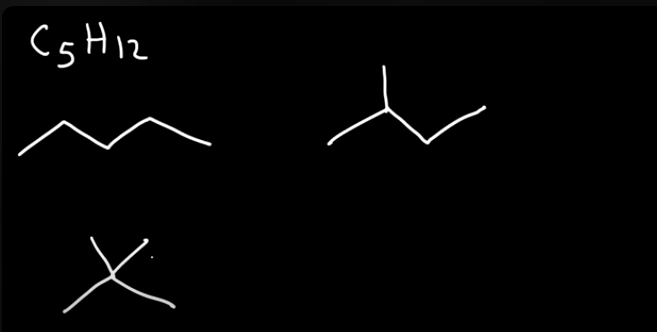
Draw all the constitutional isomers of:
C5H12
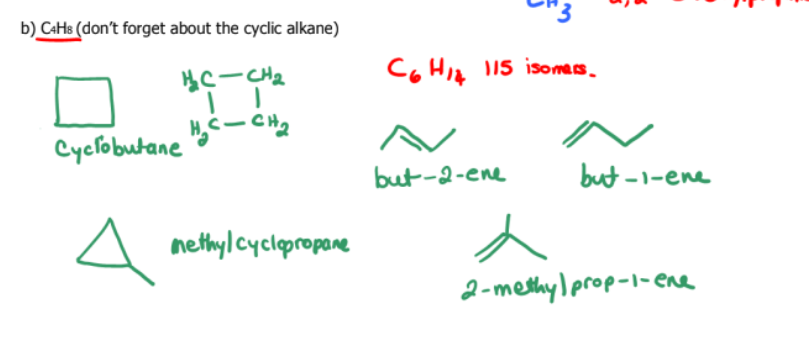
Draw all the constitutional isomers of:
C4H8
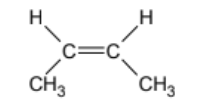
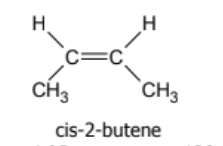
name
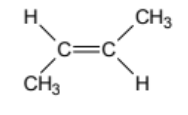
name
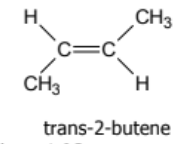
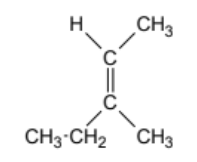
geometric isomer or not?
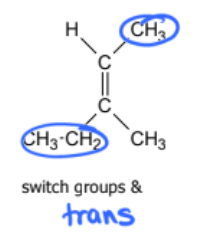
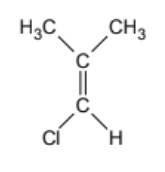
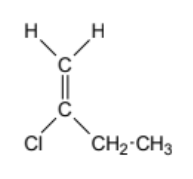
geometric isomer or not
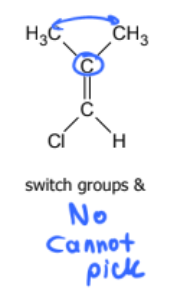
geometric isomer
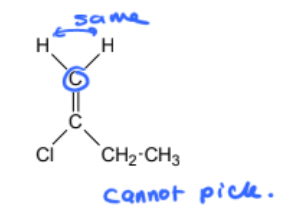
1) determine whether geometric isometric is possible
2) name the molecule
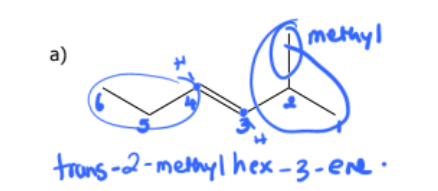
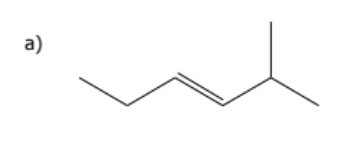
1) determine whether geometric isometric is possible
2) name the molecule
cis-4-chlorohex-2-ene
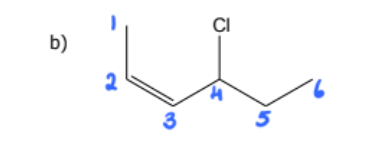
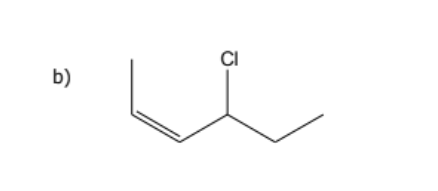
1) determine whether geometric isometric is possible
2) name the molecule
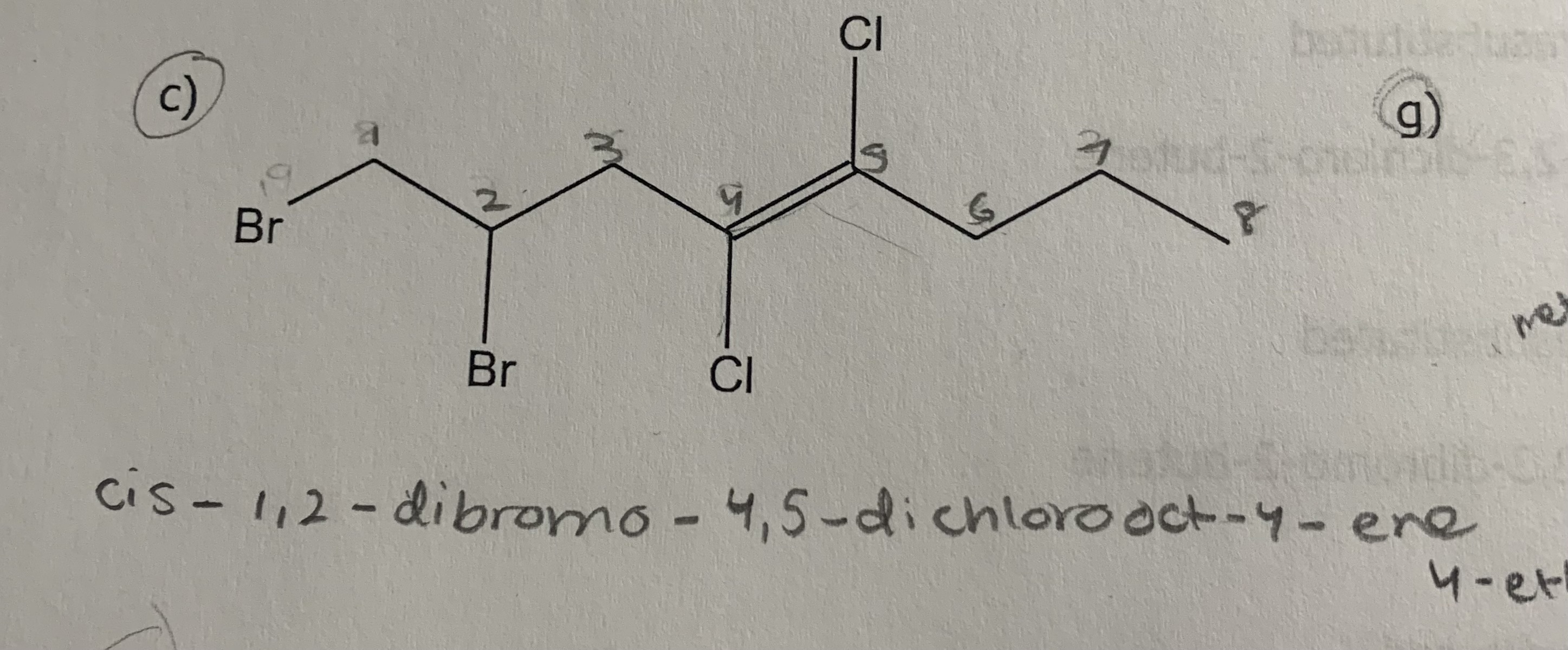
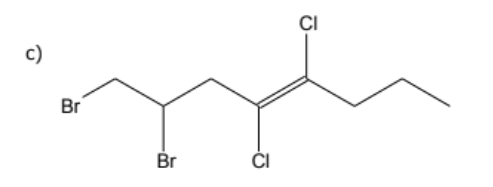
1) determine whether geometric isometric is possible
2) name the molecule
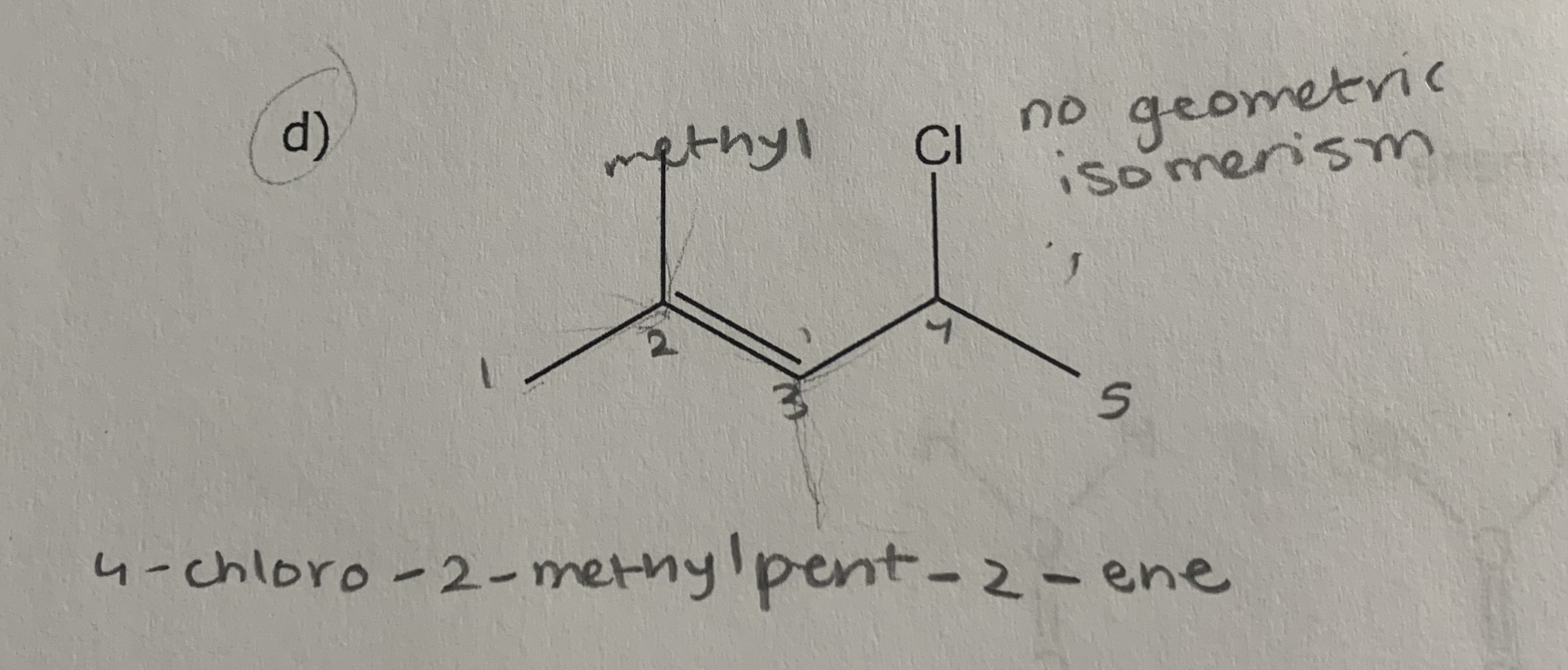
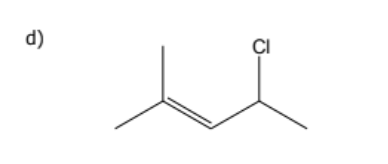
1) determine whether geometric isometric is possible
2) name the molecule
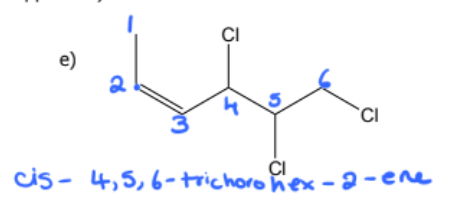
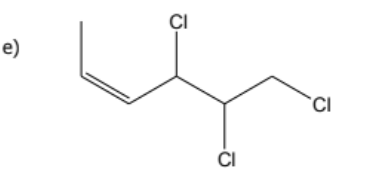
1) determine whether geometric isometric is possible
2) name the molecule
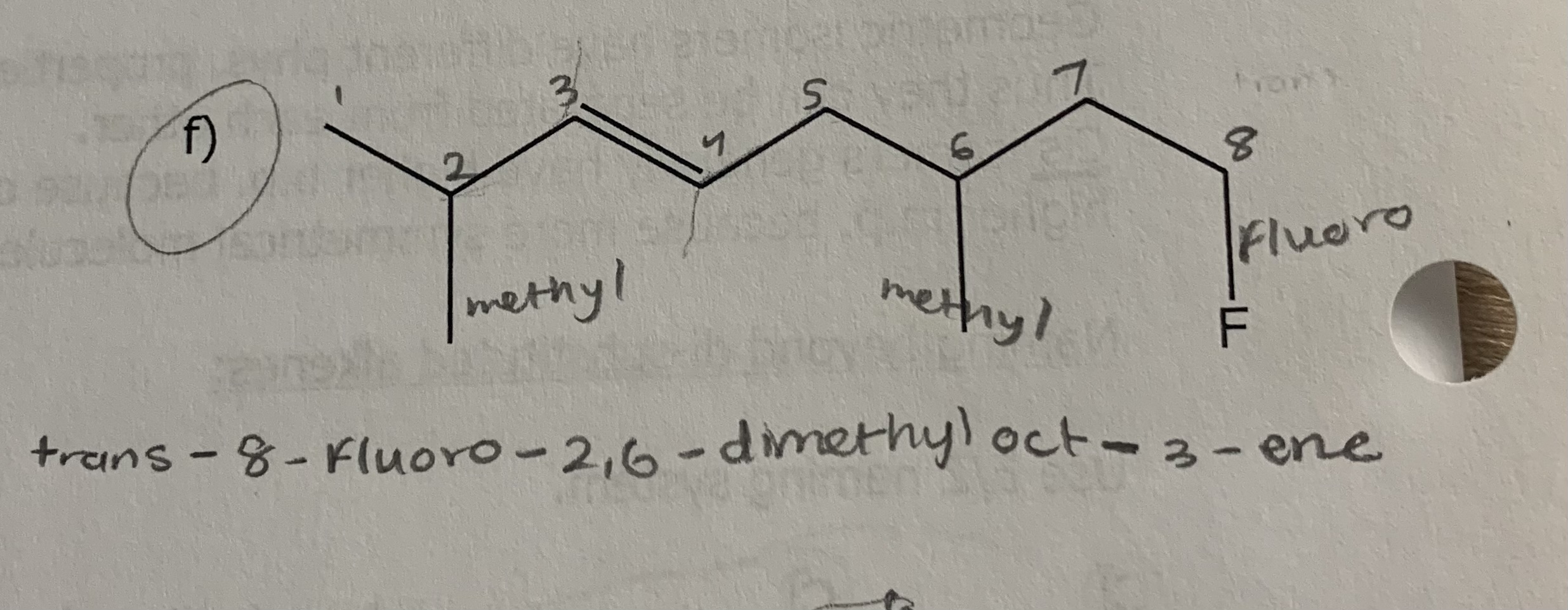
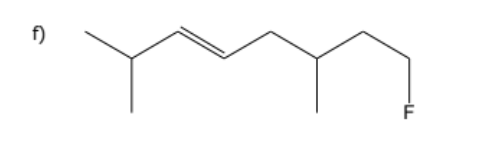
1) determine whether geometric isometric is possible
2) name the molecule
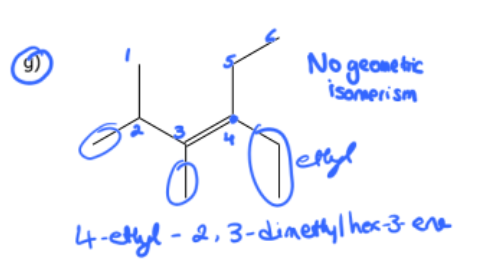
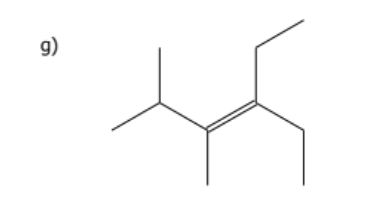
1) determine whether geometric isometric is possible
2) name the molecule
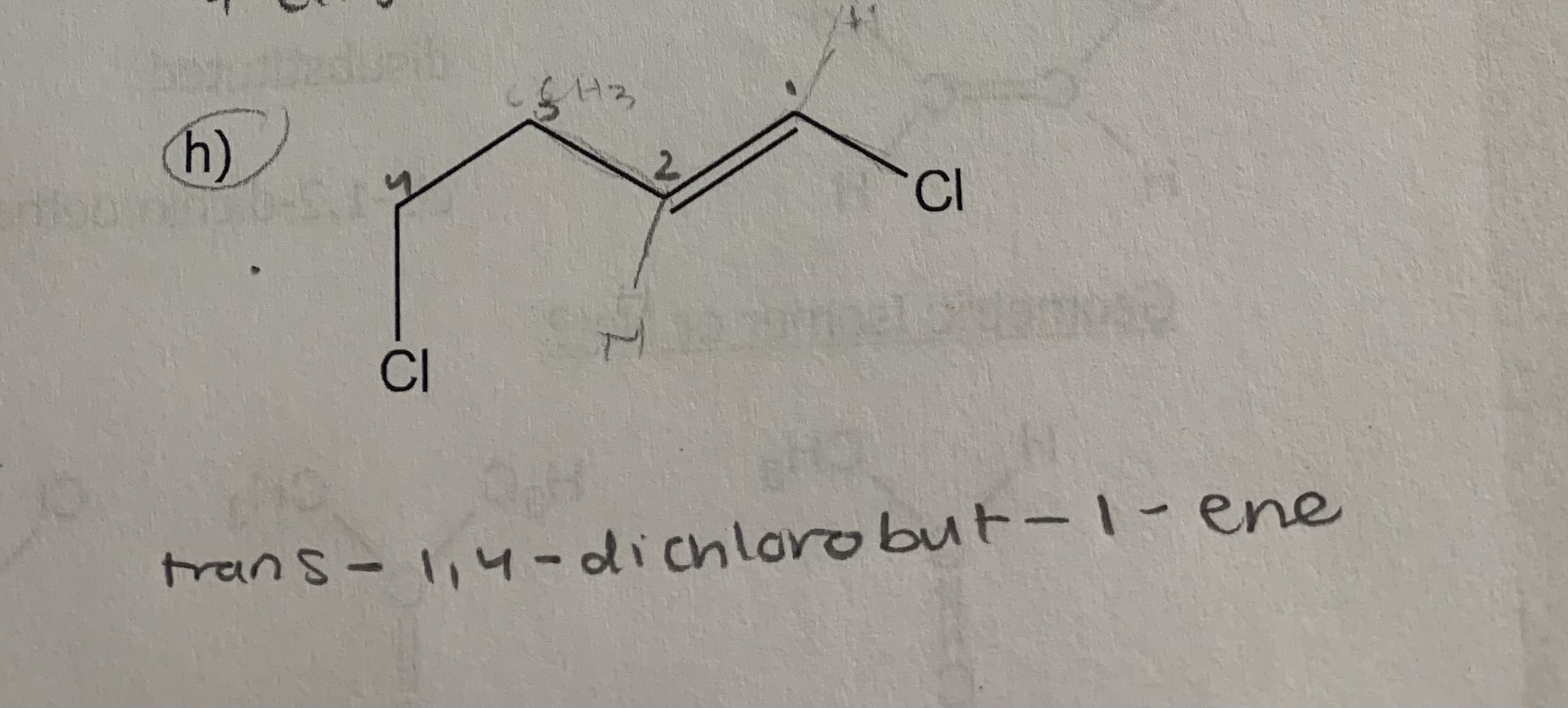
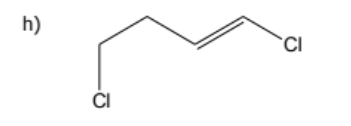
draw:
cis-1,1,1-trichloro-5-methylhex-3-ene
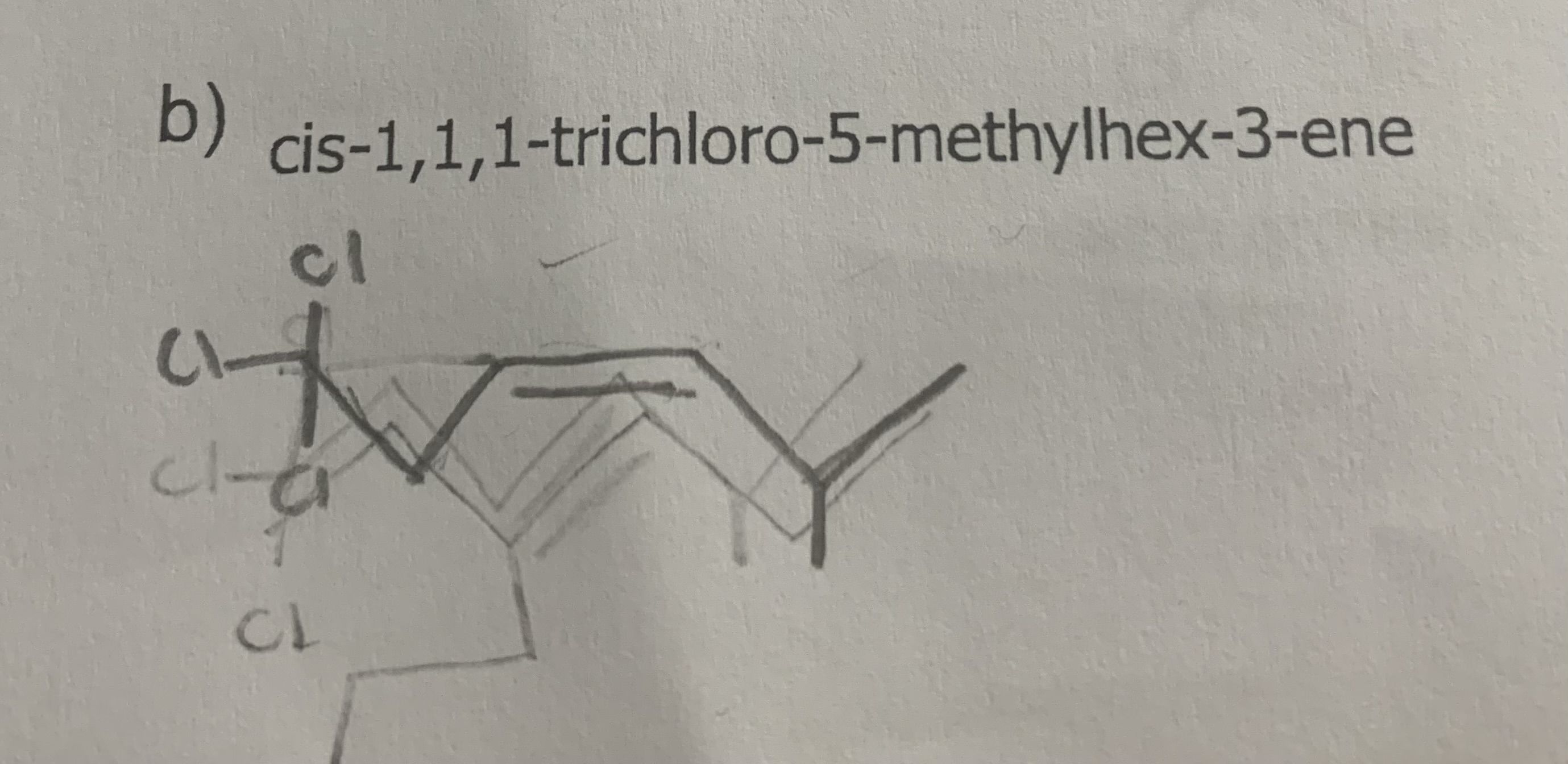
Why is the name
cis-4-bromo-2-methylpent-2-ene
incorrect?
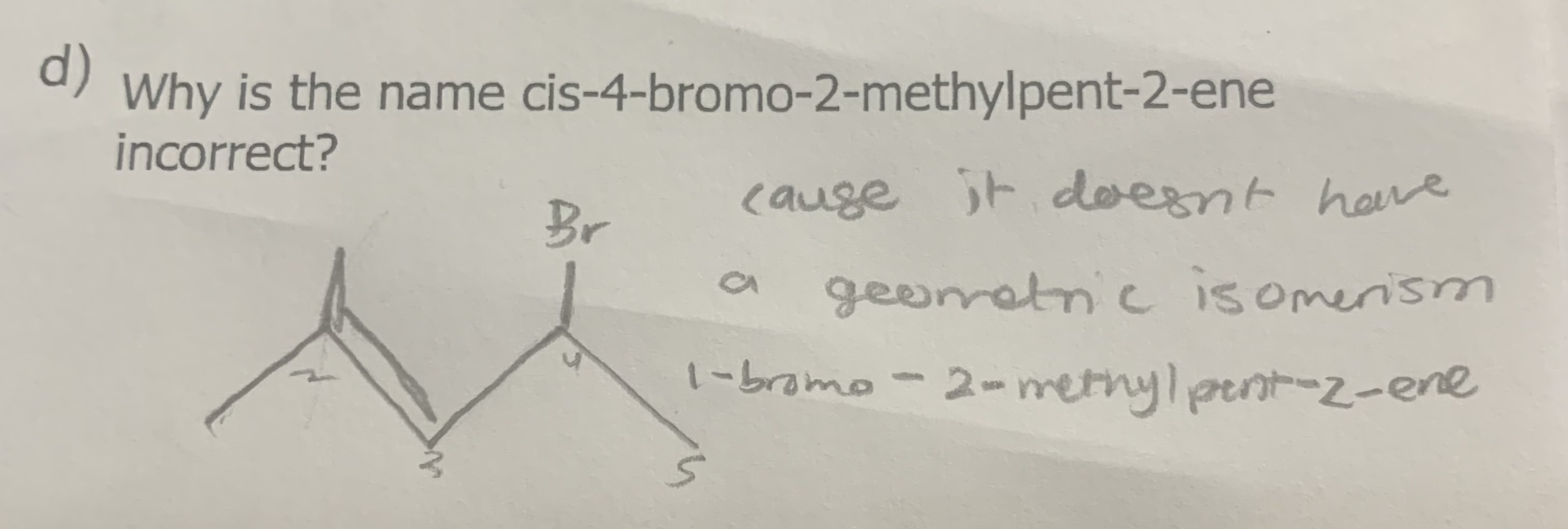
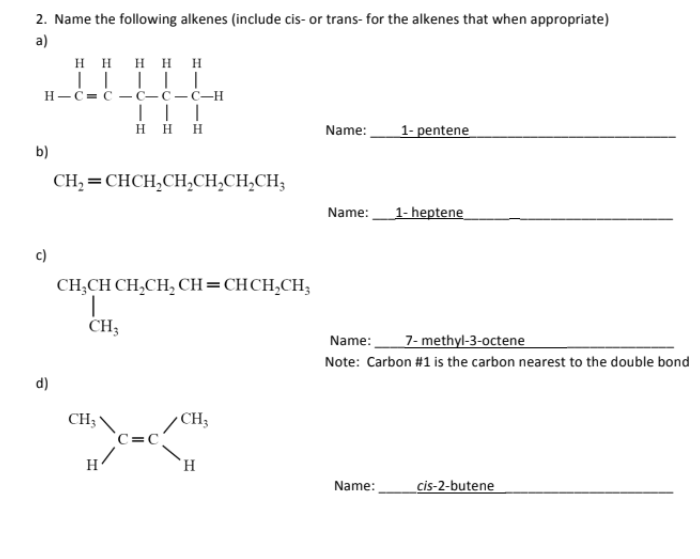
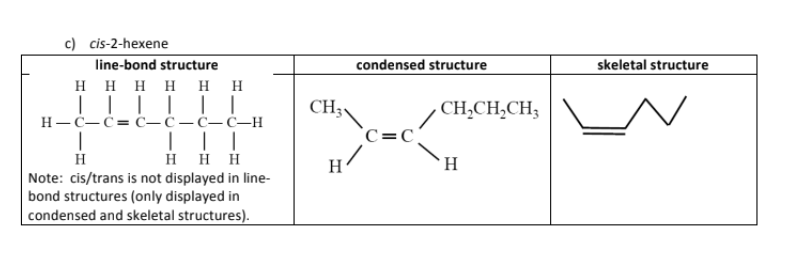
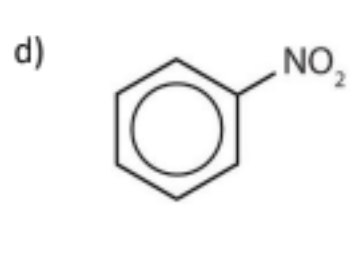
name
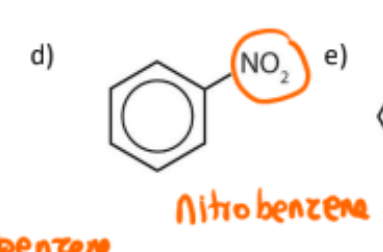
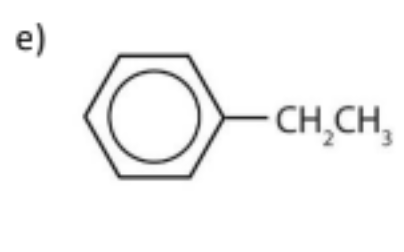
name
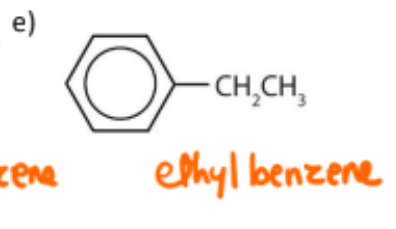
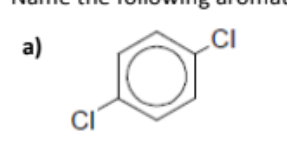
name
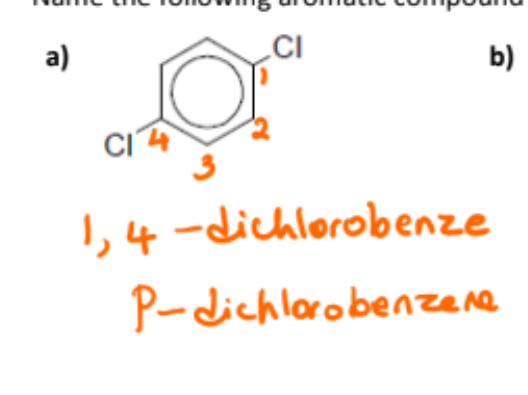
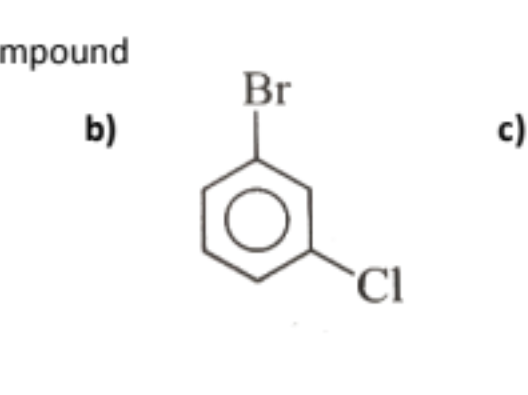
name
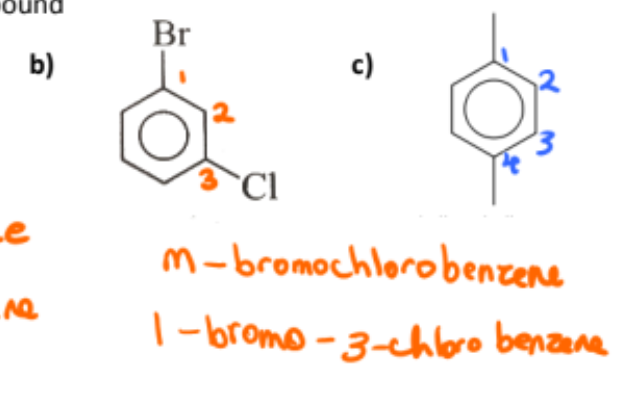
name
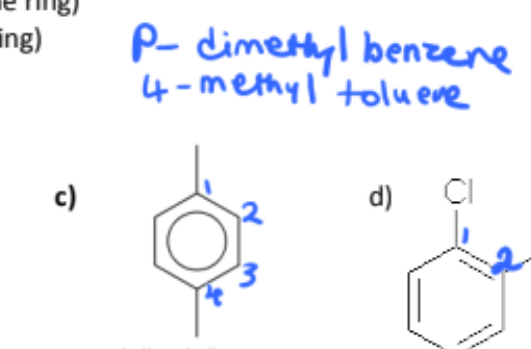
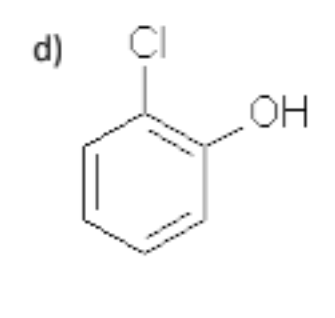
name

draw

draw

draw

draw

draw

draw

draw
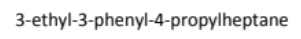
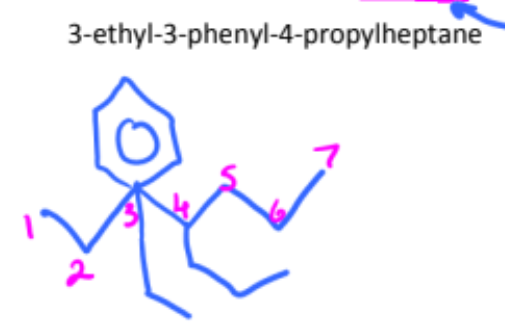
draw
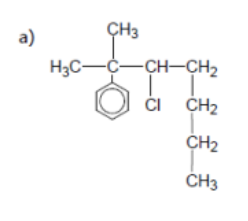
name
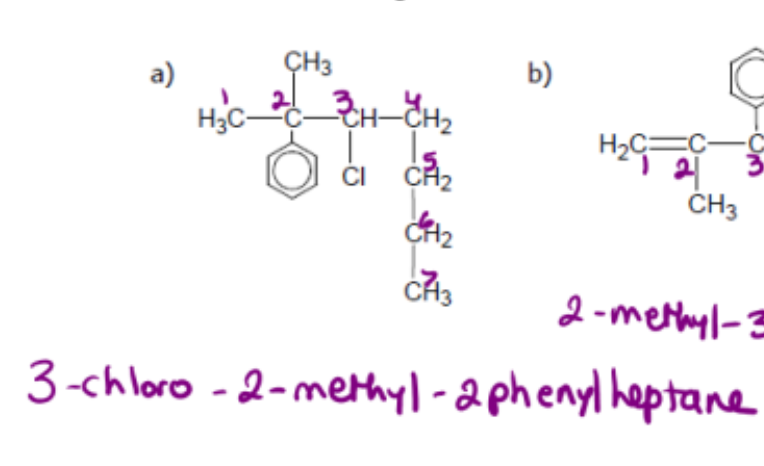
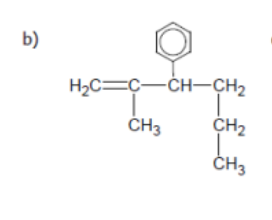
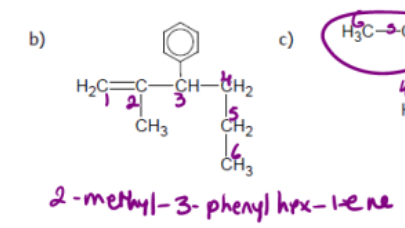
name
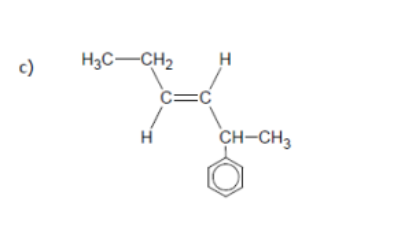
name
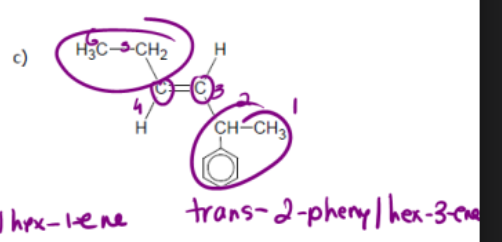

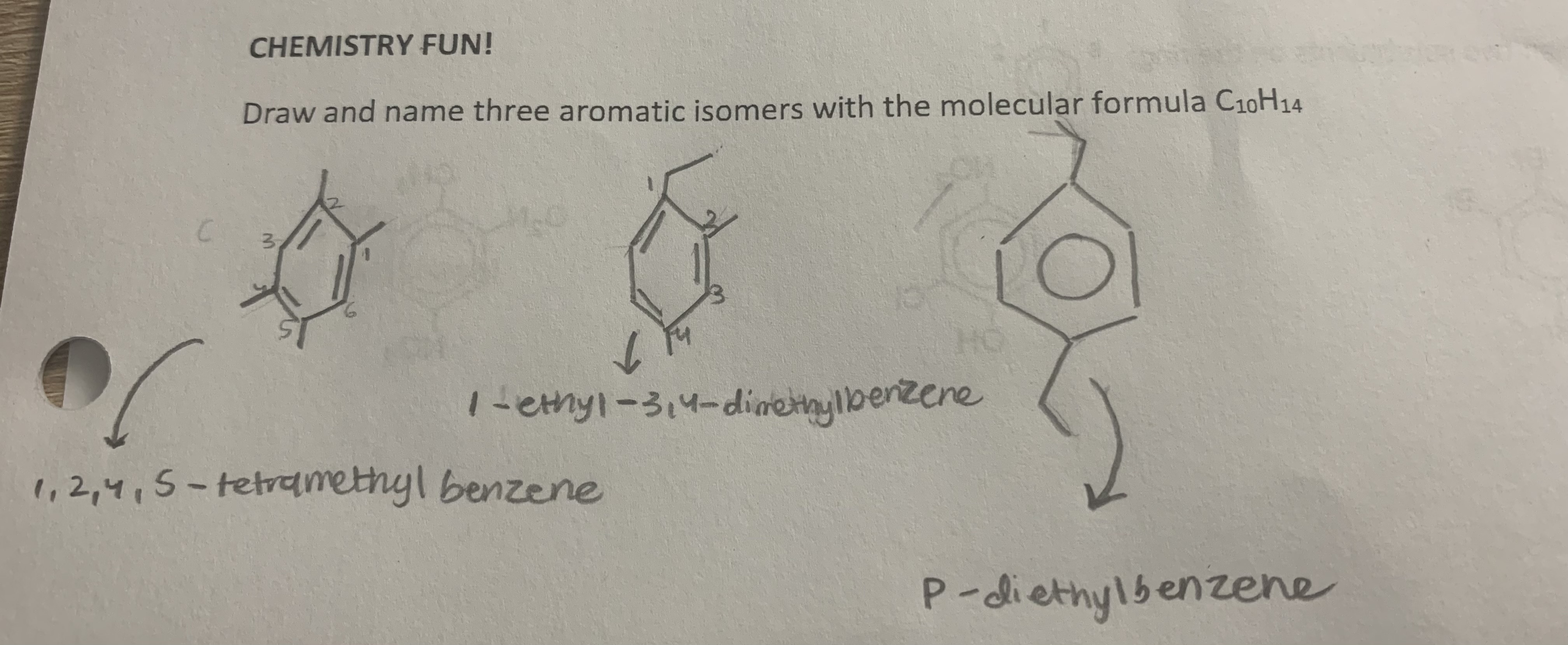
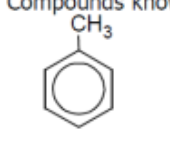
name
toluene
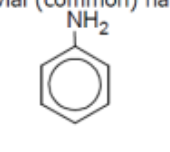
name
aniline
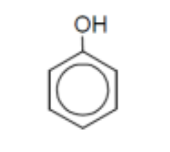
name
phenol
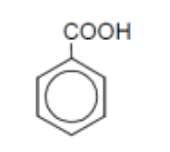
name
benzoic acid
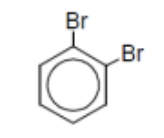
name
o-dibromobenzene
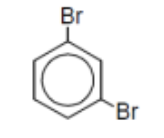
name
m-dibromobenzene
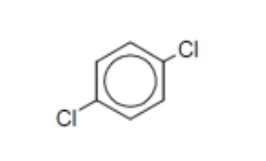
name
p-dichlorobenzene
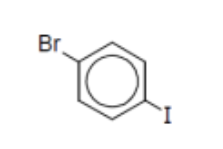
name
p-bromoiodobenzene
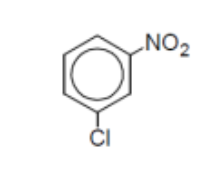
name
m-chloronitrobenzene
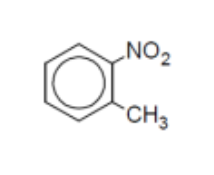
name
o-methylnitrobenzene
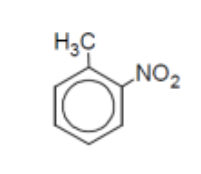
name
o-methylnitrobenzene
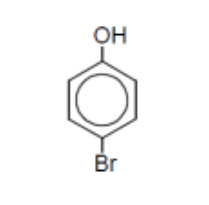
name
4-bromophenol
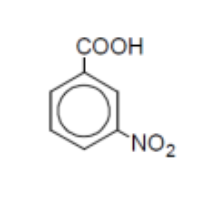
name
3-nitrobenzoic acid
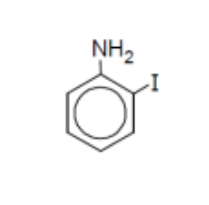
name
2-iodoaniline
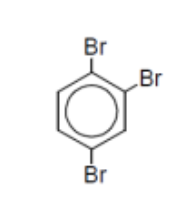
name
1,2,4-tribromobenzene
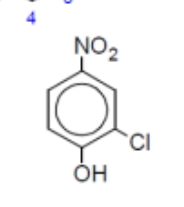
name
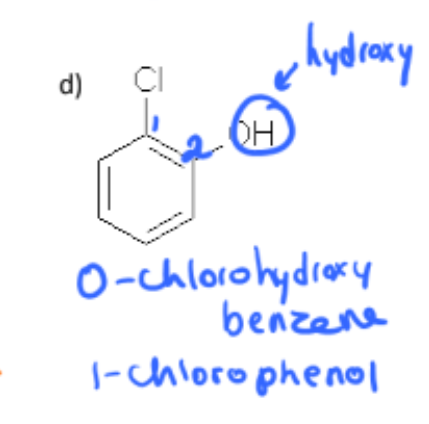
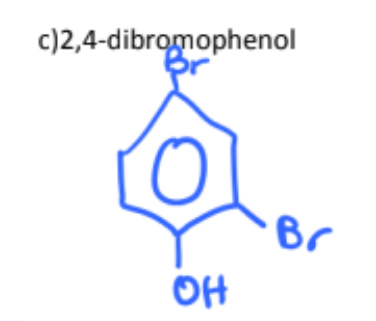
2-chloro-4-nitrophenol
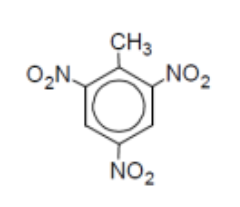
name
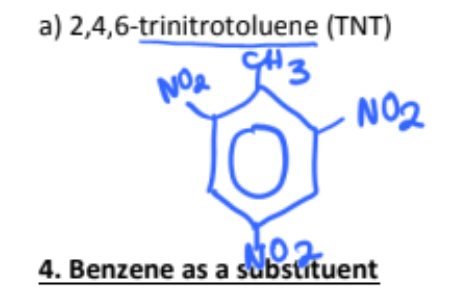
2,4,6-trinitrotoluene
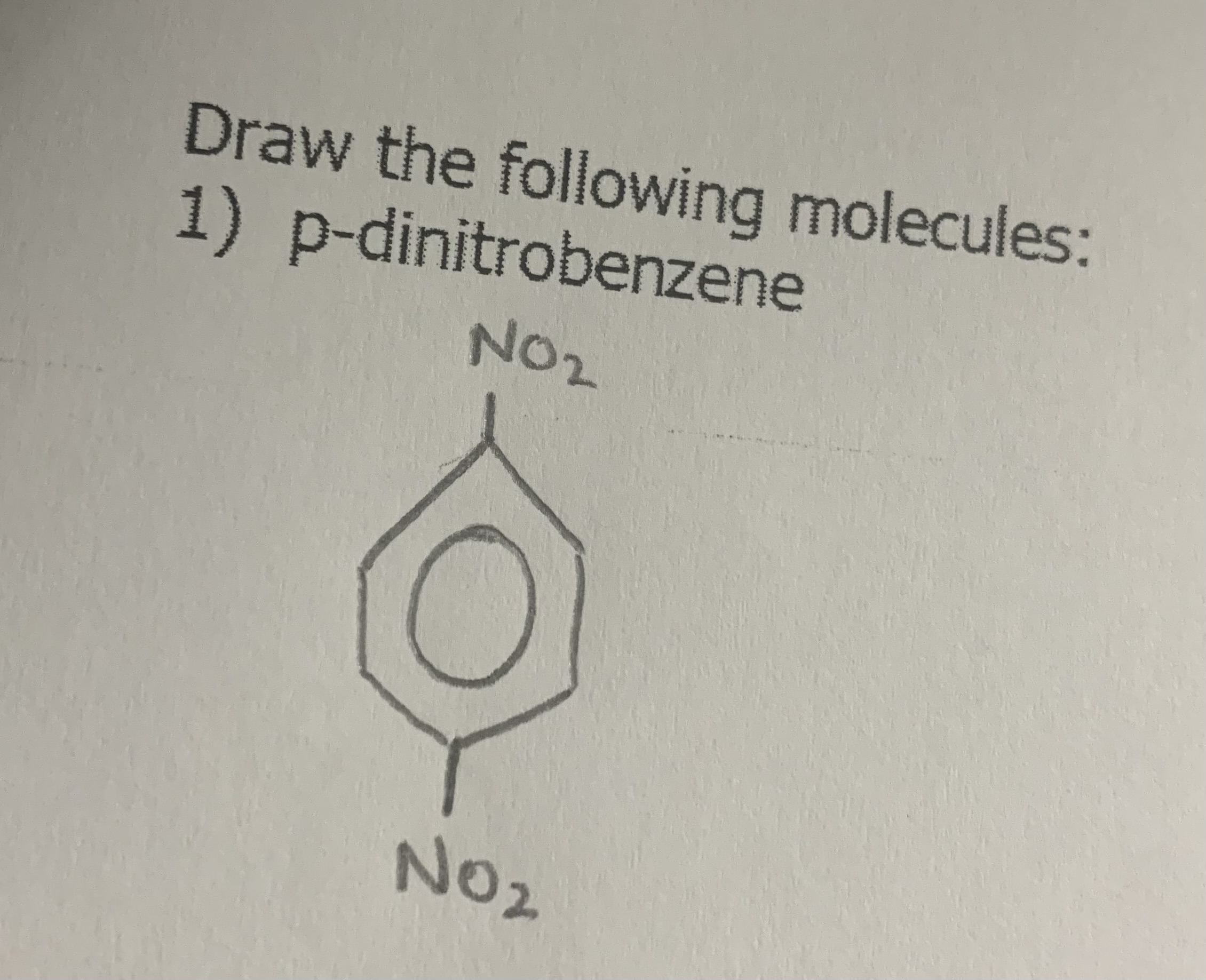
Draw:
p-dinitrobenzene
draw:
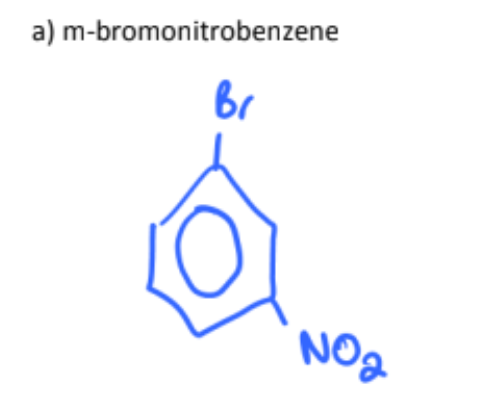
m-bromonitrobenzene
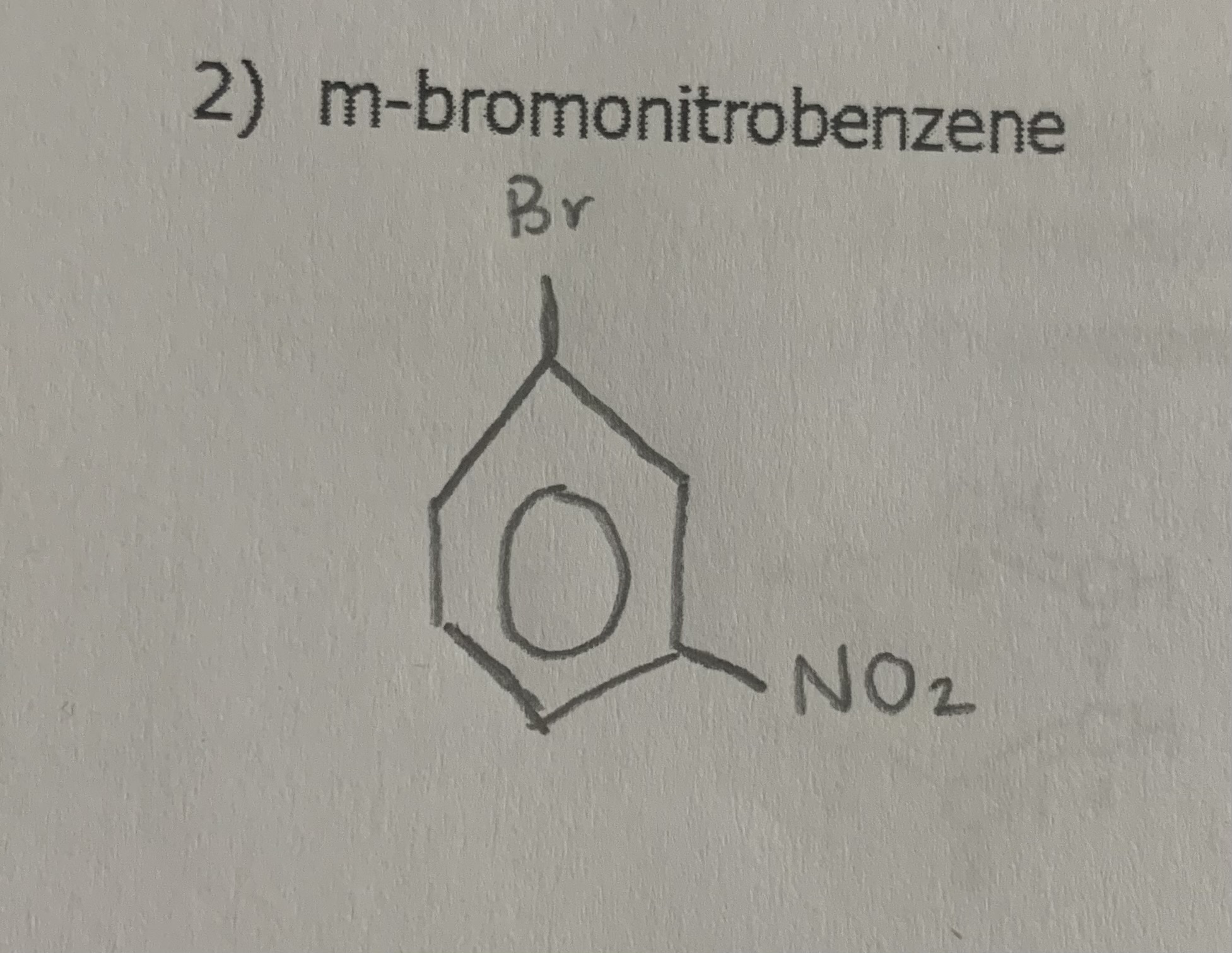
draw:
o-chlorobenzoic acid
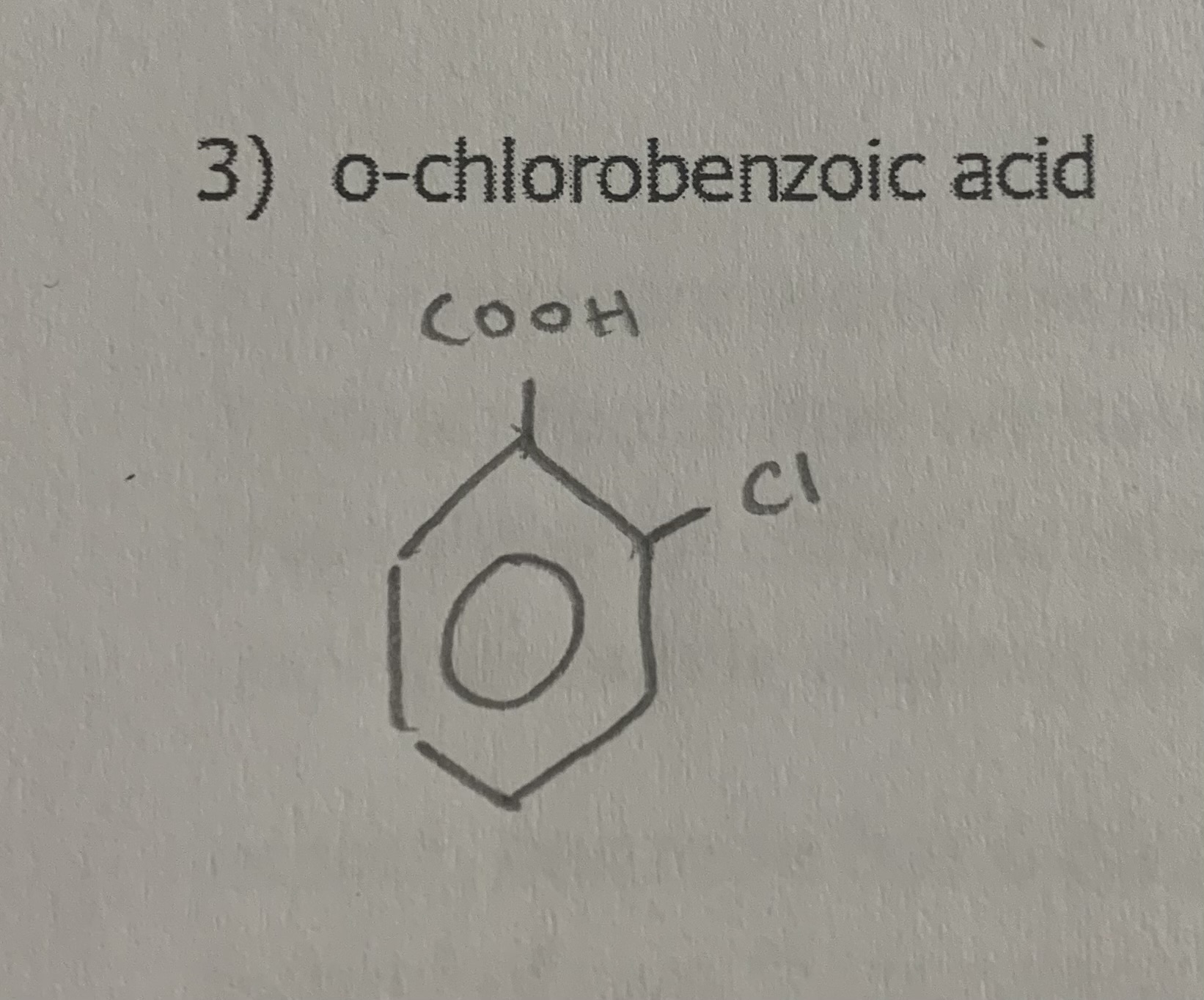
draw:
m-nitrotoluene
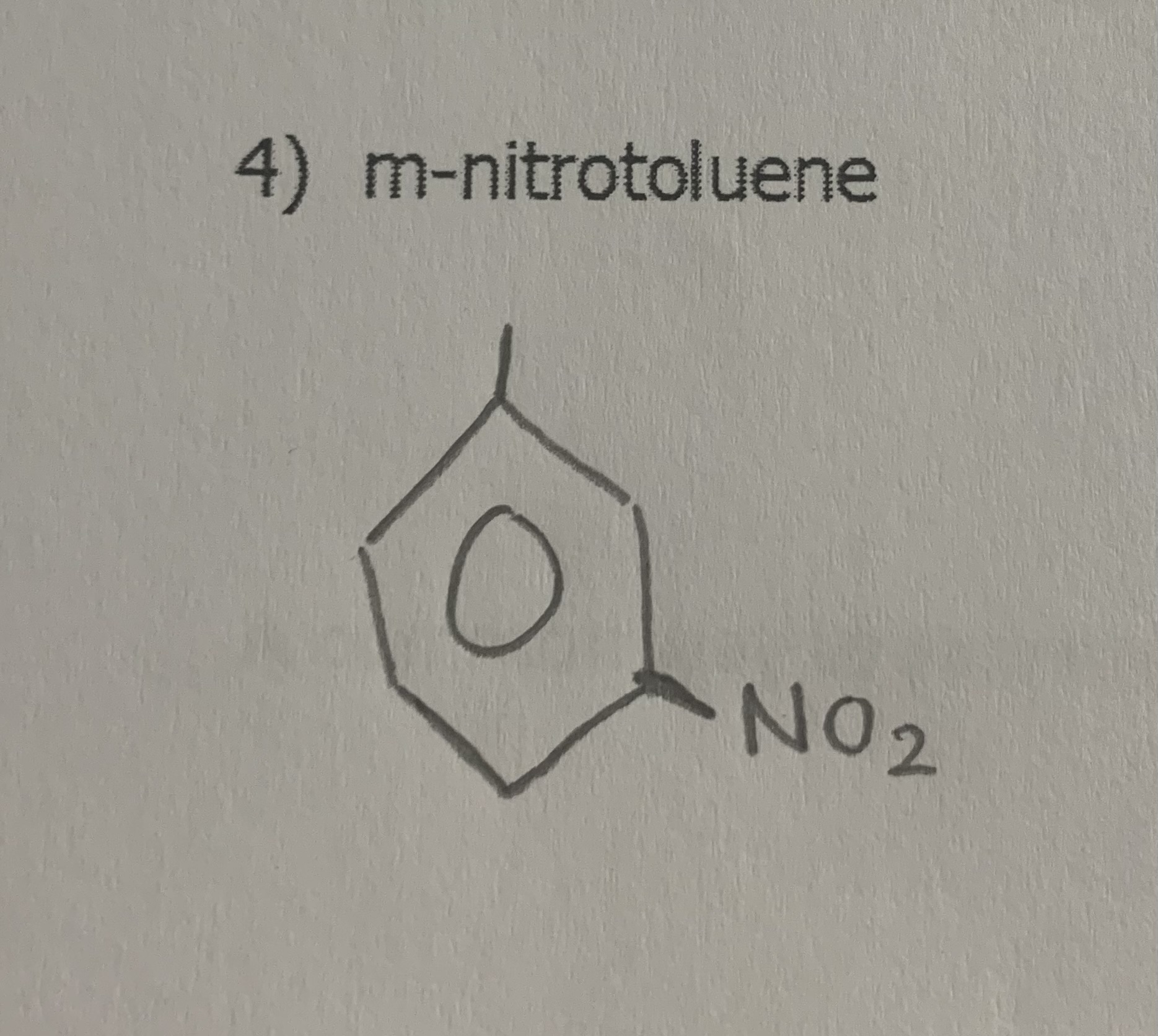
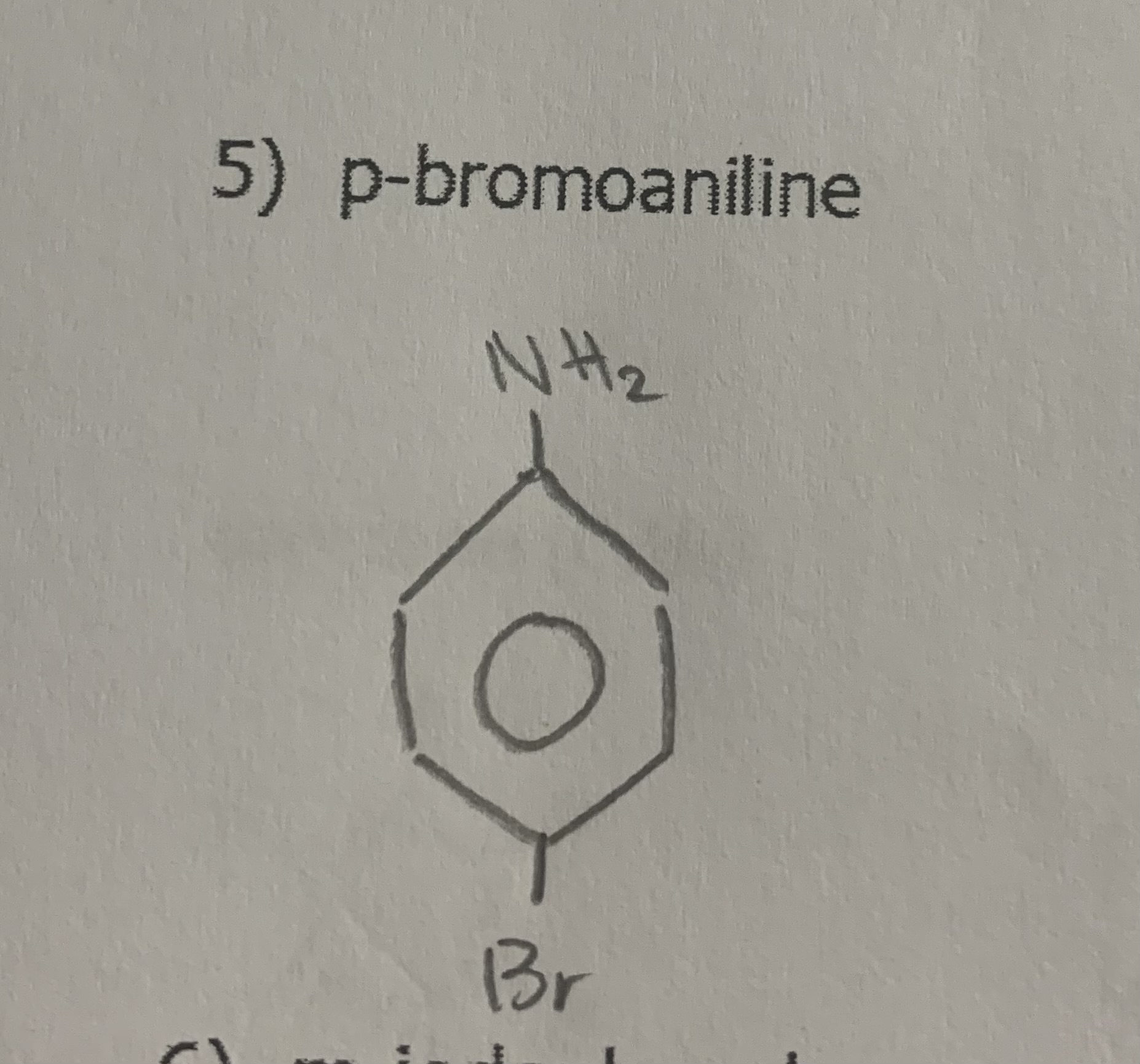
draw:
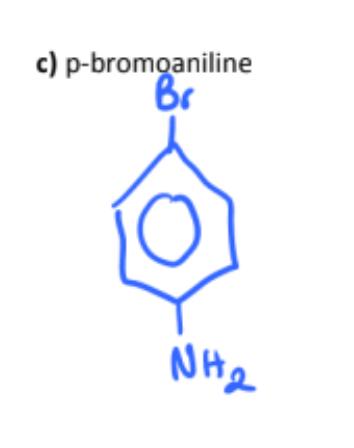
p-bromoaniline
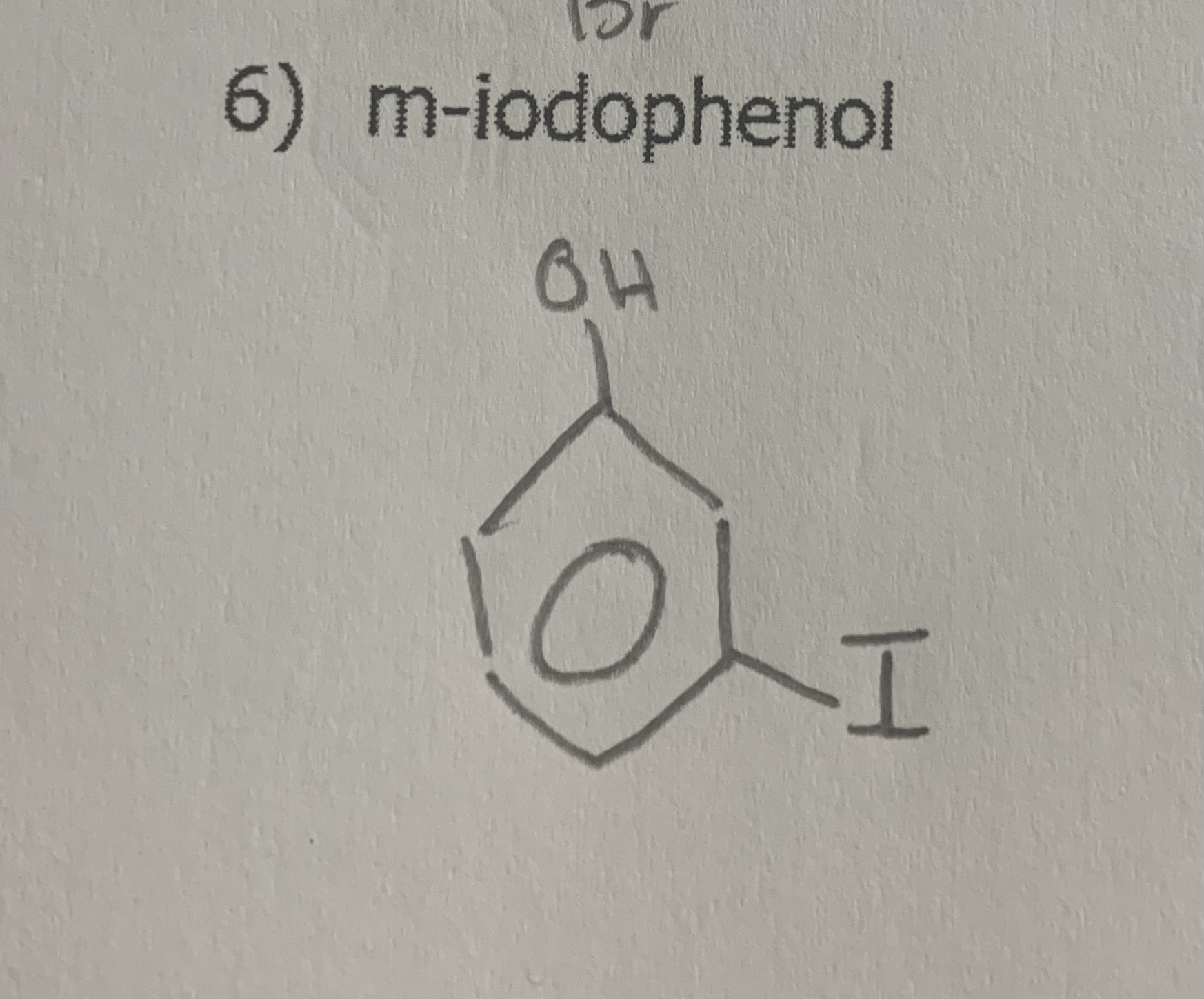
draw:
m-iodophenol
draw:
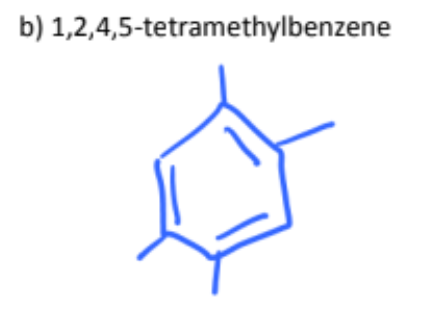
1,3,5-trimethylbenzene
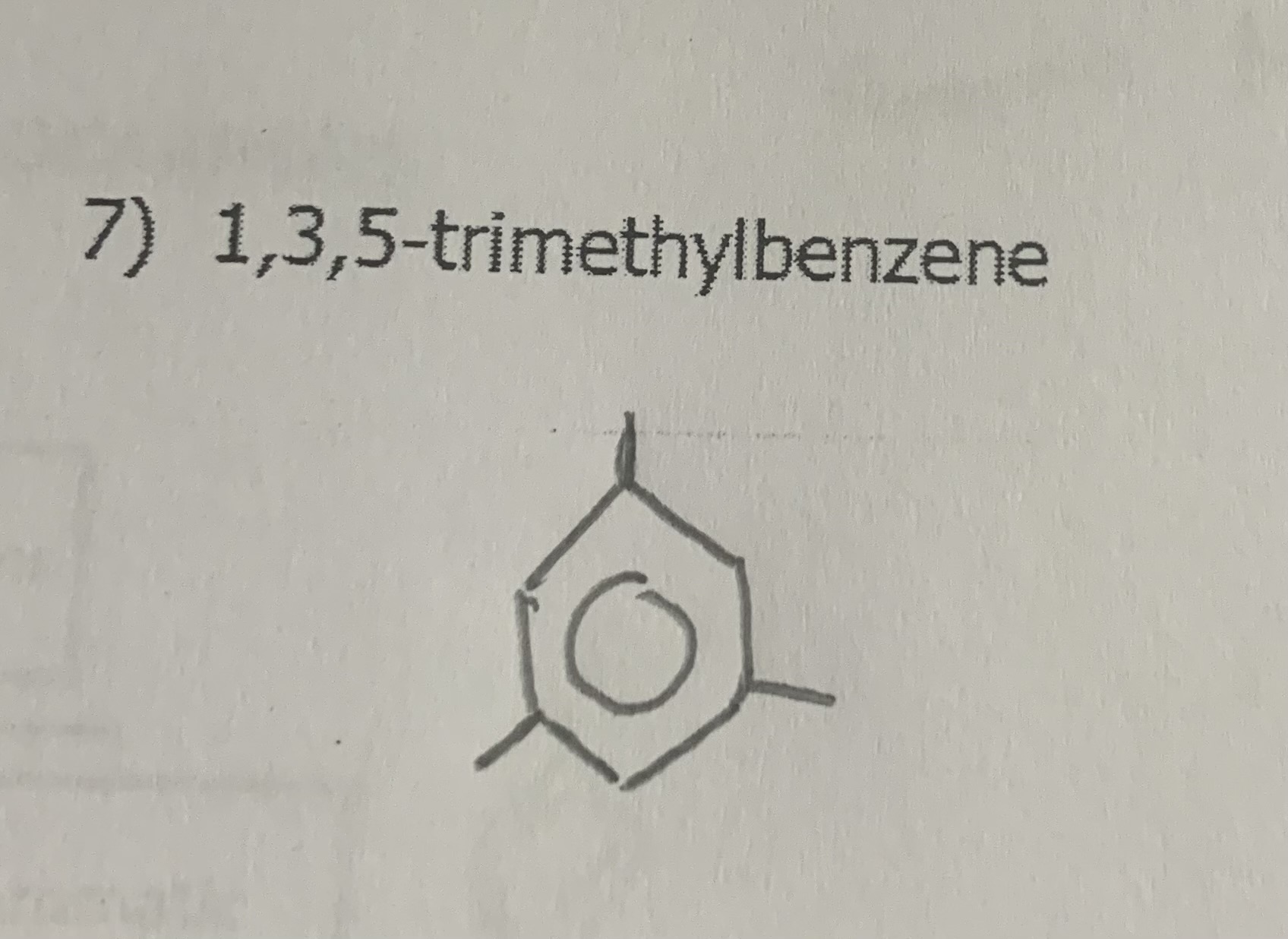
draw:
4-chloro-2,3-dinitrotoluene
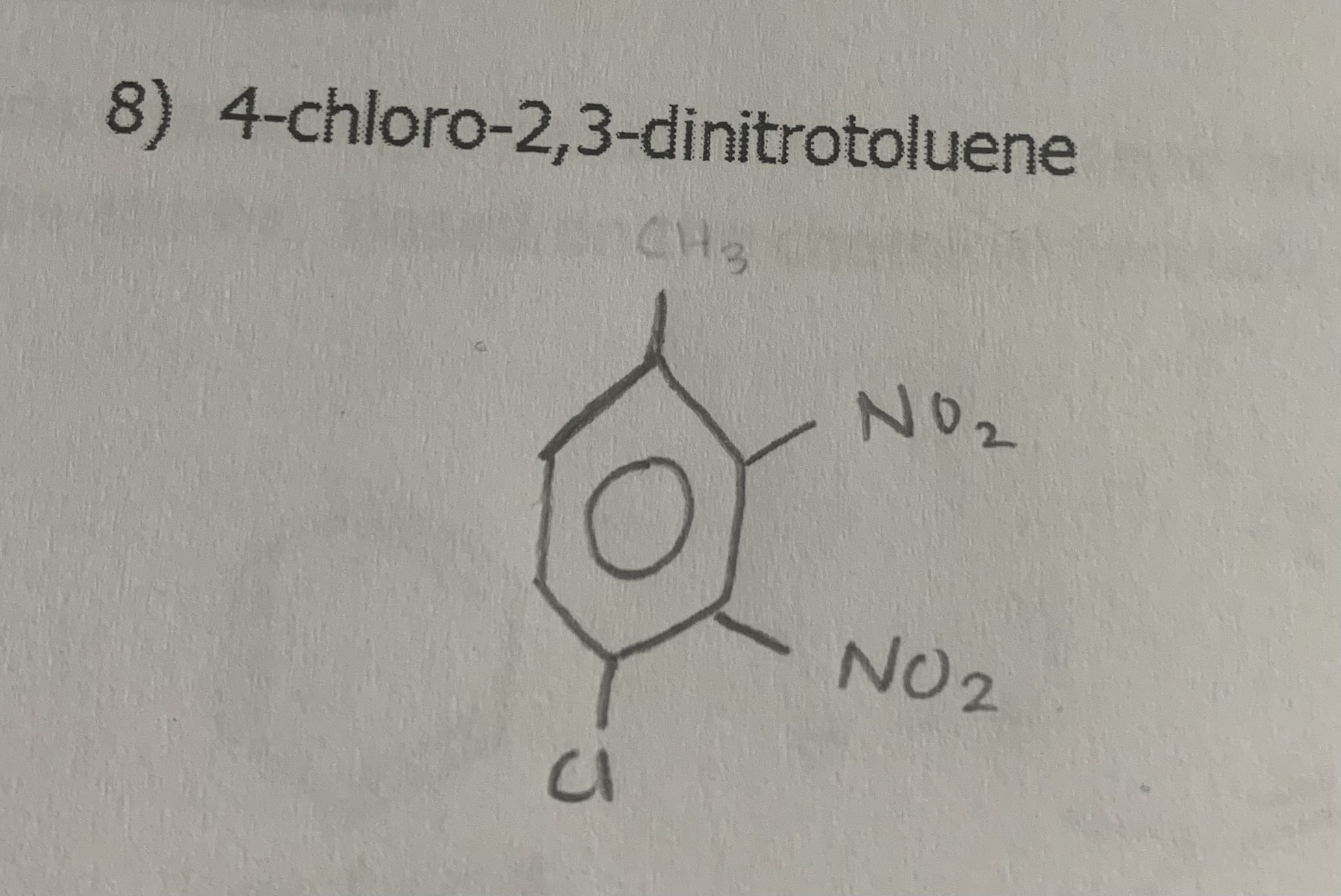
draw:
2-amino-5-bromo-3-nitrobenzoic acid
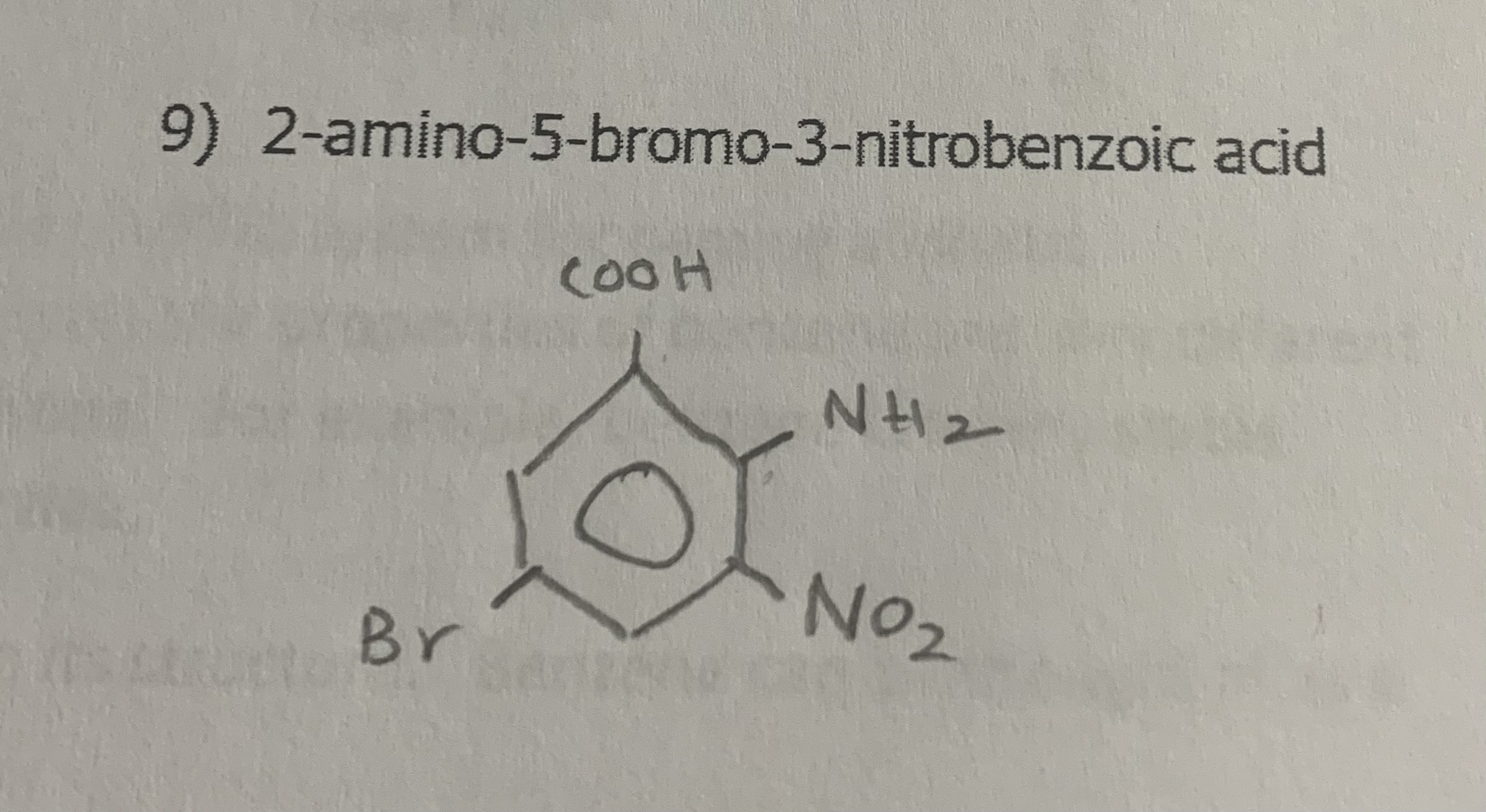
draw:
2,4,6-trinitrophenol
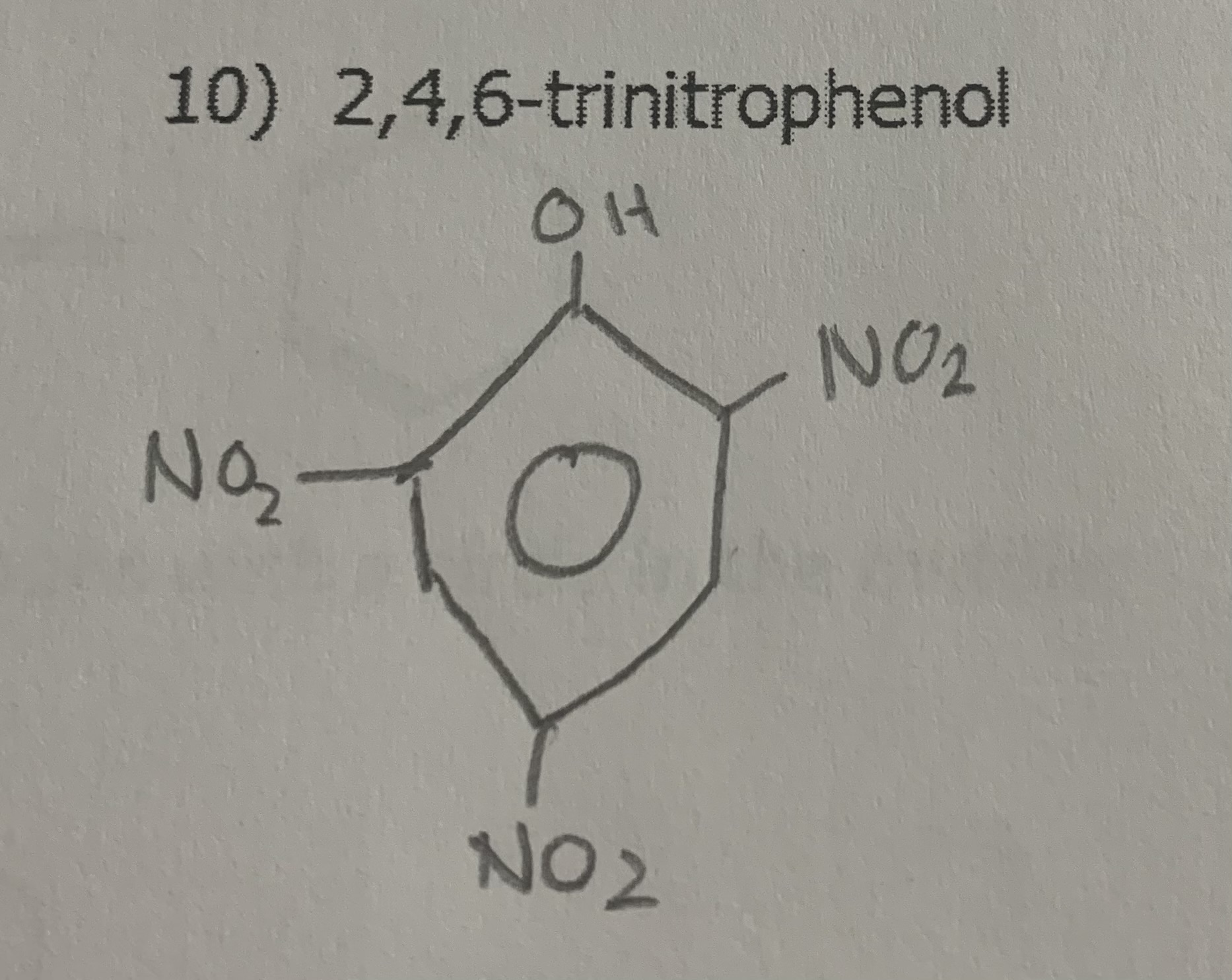
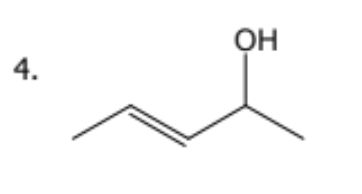
name
trans-pent-3-en-2-ol.
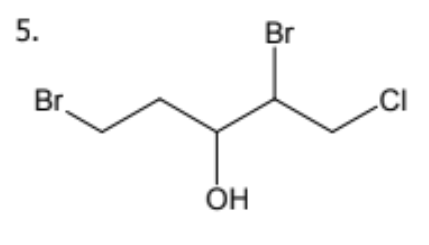
name
2-5-dibromo-1-chloropentan-3-ol