rate of reaction
1/21
Earn XP
Description and Tags
week 4, question 2 + 4
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
A reaction between two particles can only occur if the particles collide with
sufficient energy and correct orientation
activation energy
minimum energy needed for a collision to be successful
increasing temp on rate
more kinetic energy = more frequent collisions
more energy > Ea = more successful collisions
increased rate
increasing conc on rate
more particles in a unit volume
more FREQUENT SUCCESSFUL collisions
increased rate
increasing pressure on rate
particles are pushed closer together
more FREQUENT SUCCESSFUL collisions
increased rate
increasing SA of solid on rate
more particles on the surface are exposed + available
for FREQUENT SUCCESSFUL collisions
increased rate
adding a catalyst on rate
alt pathway with lower Ea provided
increased rate
rate =
[conc] / time
rate units
mol dm-3
steeper line
faster reaction
zero order rate-conc graph
horizontal line
first order rate-conc graph
directly proportional
second order rate-conc graph
curve
what affects the value of K
temp and catalyst
rate equation
rate = k [A]n [B]m
overall order =
sum of the powers
when does initial rate start
t = 0
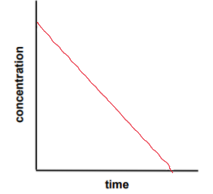
zero order
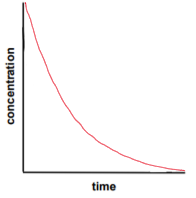
first order
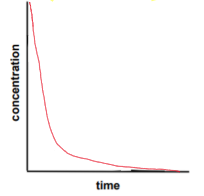
second order
half life
the amount of time needed for a reactant concentration to decrease by half
first order and half life
constant half life