Solutions Chem
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
What is a solution?
A homogeneous mixture of two or more substances. It can be a solid, liquid, or gas
what is a solute?
the substance being dissolved (generally in a lesser quantity)
what is a solvent?
The substance doing the dissolving (generally in a greater quantity)
What does like dissolve like mean?
Polar substances will only dissolve in other polar substances
what increases speed of dissolving?
heat, pressure, agitation, surface area
what does miscible mean?
when a solute and solvent are completely as one?
Will you see a meniscus in a miscible solution?
No
Will you see a meniscus in an immiscible solution?
Yes
What a meniscus?
a join between substances
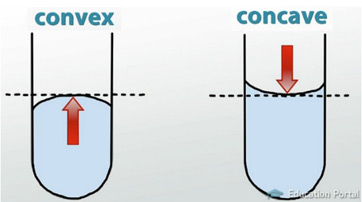
What substance has a double meniscus?
water
what does it mean if a solution is saturated?
the solution has reached the point when no more solute will dissolve in the solvent
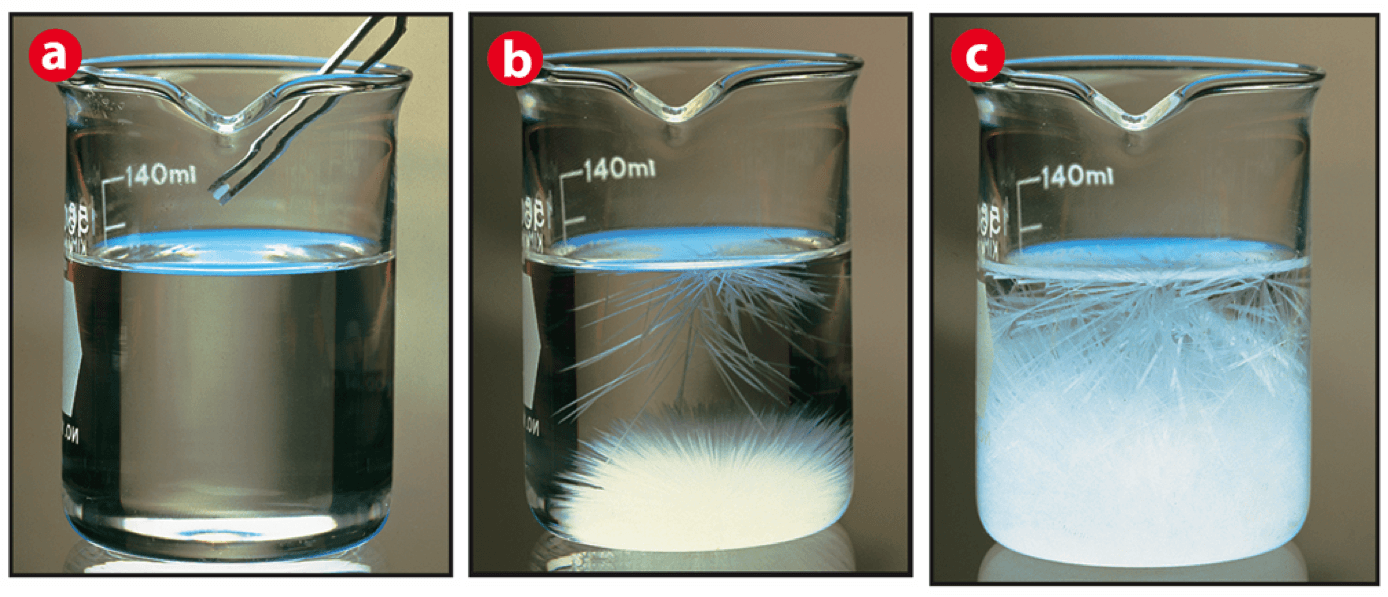
which is unsaturated, saturated, and supersaturated
A) unsaturated B) saturated C) Supersaturated
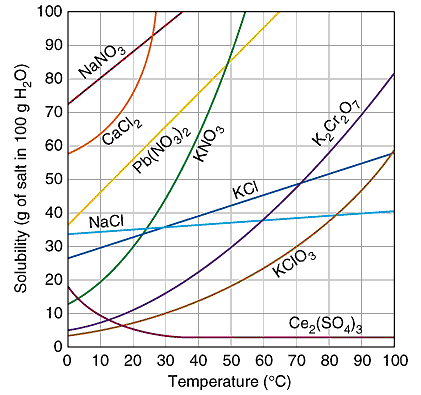
how do you read the graph?
on the line: saturated
below on the line: unsaturated
above the line: supersaturated
when a substance dissolves, ______ of the substance ______ from each other and _______ into the solution
particles separate disperse
do molecular compounds dissolve?
(generally) NO. the particles do not separate, but instead form neutral particles
What is dissociation?
Ionic dissolving
Do dissociated solutions conduct electricity?
Yes. It still has charged (free) ions, but as a whole remains neutral
What happens during dissociation?
The solute ions move apart from each other and and become randomly distributed in the solution
What is solvation?
the interaction between solute and solvent (attracting and breaking apart)
What is Ionization?
dissolving in water but ONLY with acids or bases
Ionic solutions are _____ conductive
Molecular solutions are _____ conductive
Acidic solutions are ____ conductive
always
never
always
What makes solutions more conductive?
The more ions present the more conductive the solution
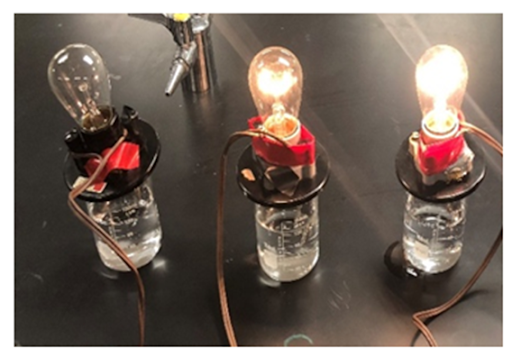
Which beaker contains the most ions?
The beaker on the right. It has the most light, therefore most conductivity, therefore most ions

what is beaker 1, 2, and 3.
1) stock solution (in pipette)
2) adding water for dilution
3) standard solution is made
what is molarity?
How many moles of a solute are in a given quantity of solution (Molarity = chemical amount)
______ solutes retain their states when placed in water. ____ solutes dissolve and become aqueous
Insoluble
soluble