Beer-Lambert Law
1/10
Earn XP
Description and Tags
CHEM 14BL
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
for volumetric glassware
report to the hundredth decimal place in mL (two decimal places)
advantages of beer-lambert law
rapid, easy to establish quality control measures for reliability, and lends itself to on-line monitoring
disadvantages of beer-lambert law
have to set up chemistry so that the absorption of light by the chemical species of interest will have a very different absorption profile than the other constituents in the solution
implication of beer’s law
if epsilon is constant, “A” should be directly proportional to “C”
epsilon in beer’s law is only constant if
wavelength is fixed during analysis and sample has a low concentration (absorbance)
absorbance for beer’s law falls within the range:
0.1 to 1.0
beer’s law graph at constant wavelength
starts at 0, slope is linear and is equal to epsilon x L
experimental response in beer’s law
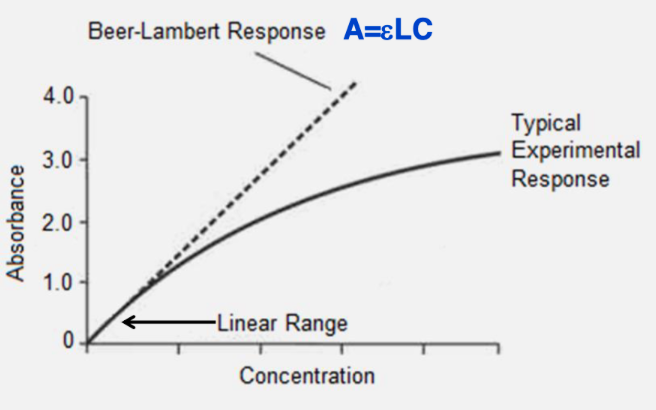
how to solve the problem: beer’s law doesn’t work if the solution concentration is too high
dilute the sample to make sure absorbance falls between 0.1 and 1
how to solve the problem: need to take care of background interference
use a blank solution to take care of this, should contain all the chemical species except the one you want to measure
in beer’s law, how can you tell if an incorrect blank solution was used?
the y intercept on the graph is not zero