BIOL 111 FINAL TAMU - FLETCHER
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
The element present in all organic molecules is
Carbon
Cell membranes have distinct inside and outside faces. Which of the following statements is the most likely explanation for the membrane's asymmetrical nature?
The two sides of a cell membrane face different environments and carry out different functions
Which of the following statements is true about buffer solutions?
They maintain a relatively constant pH when either acids or bases are added to them
When are most atoms stable?
When all of the electron orbitals in the valence shell are filled
Which two functional groups are always found in amino acids?
Carboxyl and amino groups
Nitrogen (N) is more electronegative than hydrogen (H). Which of the following is a correct statement about the atoms in ammonia (NH3)?
Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge
Which of the following is a major difference between RNA and DNA?
Type of sugar
Saturated fats ________
Contain more hydrogen than unsaturated fats that consist of the same number of carbon atoms
What component of amino acid structure varies among different amino acids
The components of the R group
The relation between amino acid and polypeptide is similar to the relation between _________
Nucleotide and nucleic acid
Which of the following is considered to be a strong base (alkali)?
NaOH → Na⁺ + OH⁻
Which statement about the sodium - potassium pump is false?
It is considered a symporter
For a protein to be an integral membrane protein, it would have to be _________
amphipathic, with at least one hydrophobic region
About 25 of the 98 natural elements are known to be essential to life. Which 4 of these 25 elements make up approximately 96% of living matter?
Carbon, hydrogen, nitrogen, oxygen
Which of the following statements correctly describes osmosis?
In osmosis, water moves across a membrane from areas of lower solute concentration to areas of higher solute concentration
Which of the following is broken when water evaporates?
Hydrogen bonds
What element does not prefer to react with other elements?
Neon
How much sodium carbonate (Na2CO3) would it take to make 1000mL of a 2M solution?
104g
What kinds of molecules pass through a cell membrane most easily?
Small and hydrophobic
If one strand of a DNA molecule has the sequence of bases 5'-ATTGCA-3', the mRNA synthesized following the template will be _____
__
3'-UAACGU-5'
Which is false regarding alpha helices and beta pleated sheets?
A beta pleated sheet forms a covalent bond between atoms on the backbone of the polypeptide
Which of the following molecules dramatically increases the rate of diffusion of water across cell membranes?
Aquaporins
What is the difference between covalent bonds and ionic bonds?
Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the transfer of electrons between charged atoms
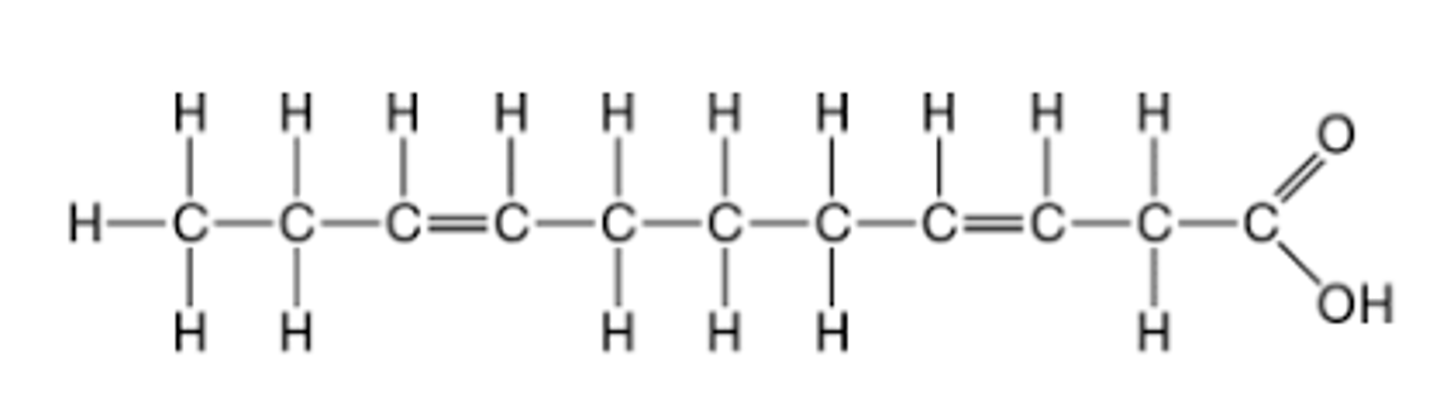
The molecule illustrated in the figure _________
will be liquid at room temperature
Which of the following best summarizes the relationship between dehydration synthesis and hydrolysis?
Dehydration synthesis reactions assemble polymers; hydrolysis reactions break polymers apart
The membranes of winter wheat are able to remain fluid when extremely cold by ___________
Increasing the percentage of cholesterol molecules in the membrane
Which two amino acids are known to have variable polarity?
Proline and tyrosine
What is the pH of a solution with a hydroxyl ion [OH-] concentration of 10-10 M?
pH 4
Which statement is true regarding a protein's primary structure?
The sequence is based upon the nucleotide sequences of the gene encoding the protein
Research indicates that ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers; that is, molecules that ______
are mirror images of each other
Which group are known as disaccharides?
Maltose, sucrose, and lactose
Which of the following processes includes all of the others?
Passive transport
A salamander relies on hydrogen bonding to stick to various surfaces. Therefore, a salamander would have the greatest difficulty clinging to a __________
Surface of hydrocarbons
Can the atomic mass of an element vary?
Yes. Adding or losing neutrons will change the atomic mass without forming a different element
An animal cell lacking carbohydrates on the external surface of its plasma membrane would likely be impaired in which function?
cell-cell recognition
Celery stalks that are immersed in fresh water for several hours become stiff. Similar stalks left in a 0.15 M salt solution become limp. From this we can deduce that the fresh water ________
Is hypotonic and the salt solution is hypertonic to the cells of the celery stalks
starch and cellulose ________
Are polymers of glucose
Why does ice float in liquid water?
Stable hydrogen bonds keep water molecules of ice farther apart than water molecules of liquid water.
Cooking oil and gasoline (a hydrocarbon) are not amphipathic molecules because they _____
Do not have polar or charged regions
Which of the following is not a polymer?
Glucose
Which of the following metabolic processes can occur without a net input of energy from some other process?
C6H12O6 + 6O2 → 6CO2 + 6H2O
The oxygen released by photosynthesis is produced by which of the following processes?
Splitting water molecules
The final electron acceptor of the electron transport chain that functions in aerobic oxidative phosphorylation is ____
oxygen
Which of the following types of reactions would decrease the entropy within a cell?
anabolic reactions
Which of the following characteristics is most likely to be associated with an enzyme that catalyzes two different chemical reactions?
Either the enzyme has two distinct active sites or the substrates involved in the two reactions have very similar structures
ATP regulates glycolysis, citric acid cycle and oxidative phosphorylation in cellular respiration through feedback control (T/F?)
False
Where are the proteins of the electron transport chain located?
mitochondrial inner membrane
The free energy for the oxidation of glucose to CO2 and water is -686 kcal/mol, and the free energy for the reduction of NAD+ to NADH is +53 kcal/mol. Why are only two molecules of NADH formed during glycolysis when it appears that as many as a dozen could be formed?
Most of the free energy available from the oxidation of glucose remains in pyruvate, one of the products of glycolysis
Early investigators thought the oxygen produced by photosynthetic plants came from carbon dioxide. In fact, it comes from _____.
Water
In the absence of oxygen, yeast cells can obtain energy by fermentation, which results in the production of which of the following sets of molecules?
ATP, CO2, and ethanol (ethyl alcohol)
Approximately how many molecules of ATP are produced from the complete oxidation of one molecule of glucose in aerobic cellular respiration?
34-38
Starting with one molecule of glucose, glycolysis results in the net production of which of the following sets of energy-containing products?
2NADH, 2 pyruvate, and 2 ATP
What happens to the free energy released as electrons are passed from photosystem II to photosystem I through a series of electron carriers?
it is used to establish and maintain a protein gradient
The light reactions of photosynthesis supply the Calvin cycle with ________
ATP and NADPH
If an enzyme is added to a solution where its substrate and product are in equilibrium, what will occur?
Nothing, the reaction will stay at equilibrium
HIV is the virus that causes AIDS. In the mid-1990s, researchers discovered an enzyme in HIV called protease. Once the enzyme's structure was known, researchers began looking for drugs that would fit into the active site and block it. If this strategy for stopping HIV infections were successful, it would be an example of what phenomenon?
Competitive inhibition
Most CO2 from catabolism is released during _____
The citric acid cycle
In chemiosmosis, what is the most direct source of energy that is used to convert ADP + Pi to ATP?
Energy released from movement of protons through ATP synthase, down their electrochemical gradient
Which of the following events are associated with chemiosmosis in chloroplasts?
The pH of thylakoid space increases and ATP is synthesized
Which process is most directly driven by light energy?
Transfer of energy from pigment molecule to pigment molecule
A chemical reaction that has a positive ΔG is best described as ______
endergonic
If pyruvate oxidation is blocked, what will happen to the levels of oxaloacetate and citric acid in the citric acid cycle shown in the accompanying figure?
Oxaloacetate will accumulate and critic acid will decrease
In mitochondria, protons move from the inter membrane space into the matrix by chemiosmosis. In chloroplasts, chemiosmosis moves protons from the _______
Thylakoid space to the membrane
Which of the following statements is an important consequence of the first law of thermodynamics for a living organism?
An organism ultimately must obtain all of the necessary energy for life from its environment
Which of the following statements describes a central role that ATP plays in cellular metabolism?
ATP provides energy coupling between exergonic and endergonic reactions
Which of the following processes generates a proton-motive force in mitochondria?
pumping of the hydrogen ions from the mitochondrial matrix across the inner membrane and into the intermembrane space
Which steps of the citric acid cycle produce NADH?
Steps 3, 4, & 8
_______ is a regulatory mechanism in which the end product of a metabolic pathway inhibits an enzyme that catalyzes an early step in the pathway.
Feedback inhibition
***picture of catabolism and cellular work***
Which of the following is the most correct interpretation of the picture?
ATP is a molecule that acts as an intermediary to store energy for cellular work
The figure shows the absorption spectrum for chlorophyll a and the action spectrum for photosynthesis. Why are they different?
Other pigments absorb light in addition to chlorophyll a
In which reactions of cellular respiration and fermentation does substrate level phosphorylation occur?
in both glycolysis and the citric acid cycle
Some photosynthetic organisms contain chloroplasts that lack photosystem II, yet are able to survive. The best way to detect the lack of photosystem II in these organisms would be
Determine whether they produce
O2 in the light
A spaceship is designed to support animal life for a multiyear voyage to the outer planets of the solar system. Plants will be grown to provide oxygen and to recycle carbon dioxide. Since the spaceship will be too far from the sun for photosynthesis, an artificial light source will be needed. Suppose a plant has a unique photosynthetic pigment and the leaves of this plant appear to be reddish yellow. What wavelengths of visible light are absorbed by this pigment?
Blue and violet
Which temperature and pH profile curves on the graphs are most likely associated with an enzyme isolated from a human stomach where conditions are strongly acid?
Curves 1&4
Which of the following events accompanies absorption of energy by chlorophyll molecules of the reaction-center complex?
an electron is excited
Which of the following statements about enzyme function is true?
Enzymes increase the rate of chemical reactions by lowering activation energy barriers
Which molecule is the final electron acceptor for electrons from photosystems I?
NADP+
Which of the following sequences describes the path by which electrons travel downhill energetically in aerobic respiration?
Glucose - NADH - electron transport chain - oxygen
Which of the following statements is true of metabolism in its entirety in all organisms?
Metabolism consists of all the energy transformation reactions in an organism
Which of the following statements describes a common characteristic of catabolic pathways?
They are exergonic and provide energy that can be used to produce ATP and ADP and Pi
The Hershey-Chase blender experiment showed that material from bacteriophage labeled with radioactive sulfur did not remain with the transacted bacteria when the phage "bodies" were knocked off. What did they conclude from this result?
Protein could not be the genetic material
Which of these represents the central dogma of genetics?
DNA -RNA -Protein
Which of these terms describes the triplets of bases that encode for an amino acid?
codon
This molecule is essential to the initiation of translation by binding to the small ribosomal subunit and eventually causing the large subunit to be recruited
eIF-2
Who utilized the x-ray cryptography image of DNA to establish that DNA is structured in a double-helix?
Watson & Crick
A skin cell differs from a heart cell in that ....
they express different sets of genes
In eukaryotes, there are several different types of RNA polymerase. Which type is involved in transcription of mRNA for a globin protein?
RNA polymerase II
Which of the following statements correctly describes the primary difference between enhancers and proximal control elements?
Enhancers are located considerable distances from the promoter; proximal control elements are closer to the promoter
Which of the following modifications is not associated with pre-mRNA?
methylation
The mitotic spindle plays a critical role in which of the following processes?
separation of sister chromatids
Which of the following processes is the first to take place in translation in eukaryotes?
the small subunit of the ribosome recognizes and attaches to the 5' cap of mRNA
A segment of DNA wrapped twice around a group of histone proteins is known as a
nucleosome
Which of the following statements correctly describes a characteristic of tumor-suppressor gene
they encode proteins that help prevent uncontrolled cell growth
Hoe does extracellular glucose inhibit transcription of the lac operon?
by reducing the levels of intracellular cAMP
Humans contain about 20,000 genes but can make 75,000-100,000 different proteins. The most likely explanation for this is
alternative splicing of exons
Which of these structures differ between prokaryotes and eukaryotes?
origin of replication
One difference between cancer cells and normal cells is that cancer cells
continue to divide even when they are tightly packed together
Rank the following statements in order of which they occur during transcription
1, 4, 3, 5, 2
Metaphase is characterized by _____.
alignment of chromosomes on the equator of the cell
Which of the following processes can occur in prokaryotes but not in eukaryotes?
transcription and translation occur simultaneously