Reaction Products and Reagents (Su
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
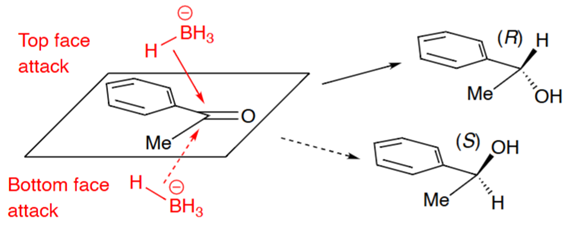
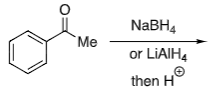
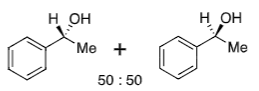
Ketone + NaBH4
50:50
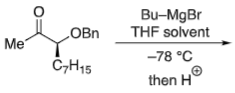
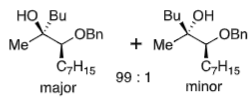
Cram chelation
heteroatom on α position
chelating metal (Mg)
New group adds to least sterically hindered side on chelated transition state
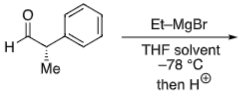
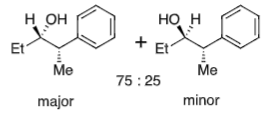
Felkin-Anh (no heteroatom)
No α position heteroatom
Conformation is fixed by bulky Ph group
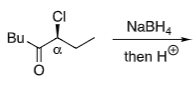
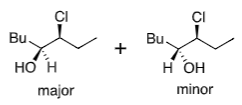
Felkin-Anh (with heteroatom):
Cl in α position
No chelating metal (Na+ non-chelating)
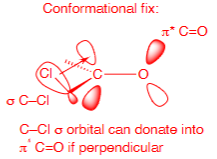
Conformational fix from most electronegative atom favouring 90o to the C=O
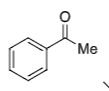
+ (S)-CBS catalyst
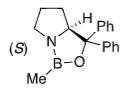
+ BH3
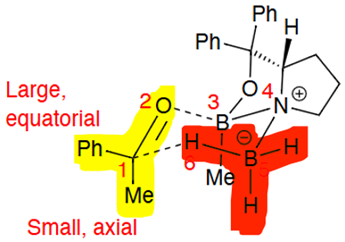
Transition state conformational fix from bulky group orientation on the 6 membered ring (structure not to be memorised)
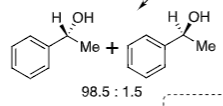
(S) catalyst adds H to the front (when the larger group is drawn on the left)
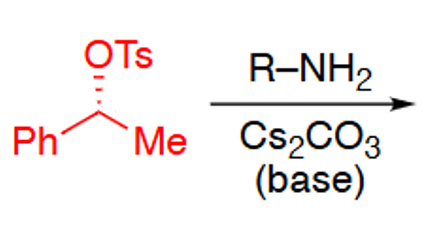
(or R-SH)
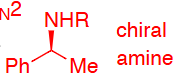
SN2 mechanism so inversion of stereochemistry
Sulfonate is a good leaving group so promotes this reaction (wouldnt react with unadapted alcohol precursor)
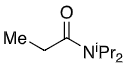
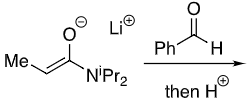
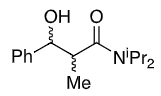
+ LDA
+ Et-I
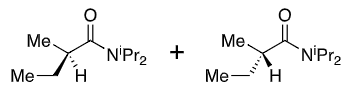
50:50
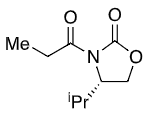
+ LDA
+ Et-I
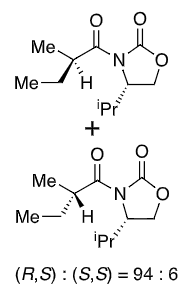
New group adds opposite side to iPr (when drawn this way)
Li chelates the enolate oxygen and carbonyl
Enolate favours cis
What reagents to produce:
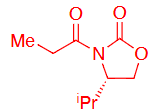
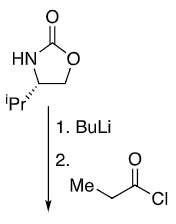
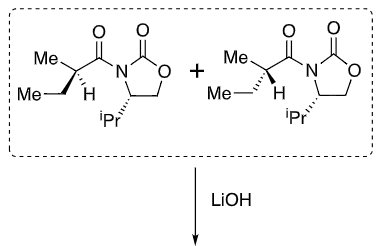
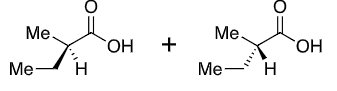
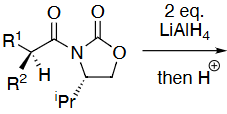
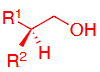
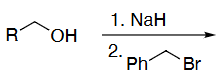
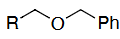
OH protection method
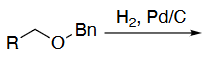
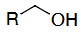
OH deprotection
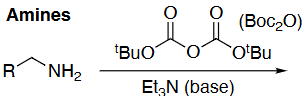
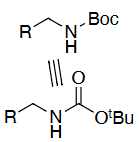
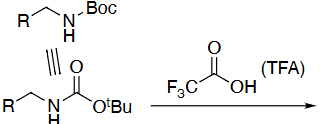
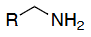
Amine deprotection