Elements, Molecules, Compounds, and Phase Changes: Key Terms and Definitions
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
absolute zero
The temperature at which all atoms and molecules stop vibrating. O Kelvin or -273.15 °C
atom
The smallest particle of an element
BEC - Bose-Einstein Condensate
State of matter that has the least amount of energy.
Atoms and molecules stop vibrating independently and move together like one giant atom.
Boiling
vaporization of a liquid
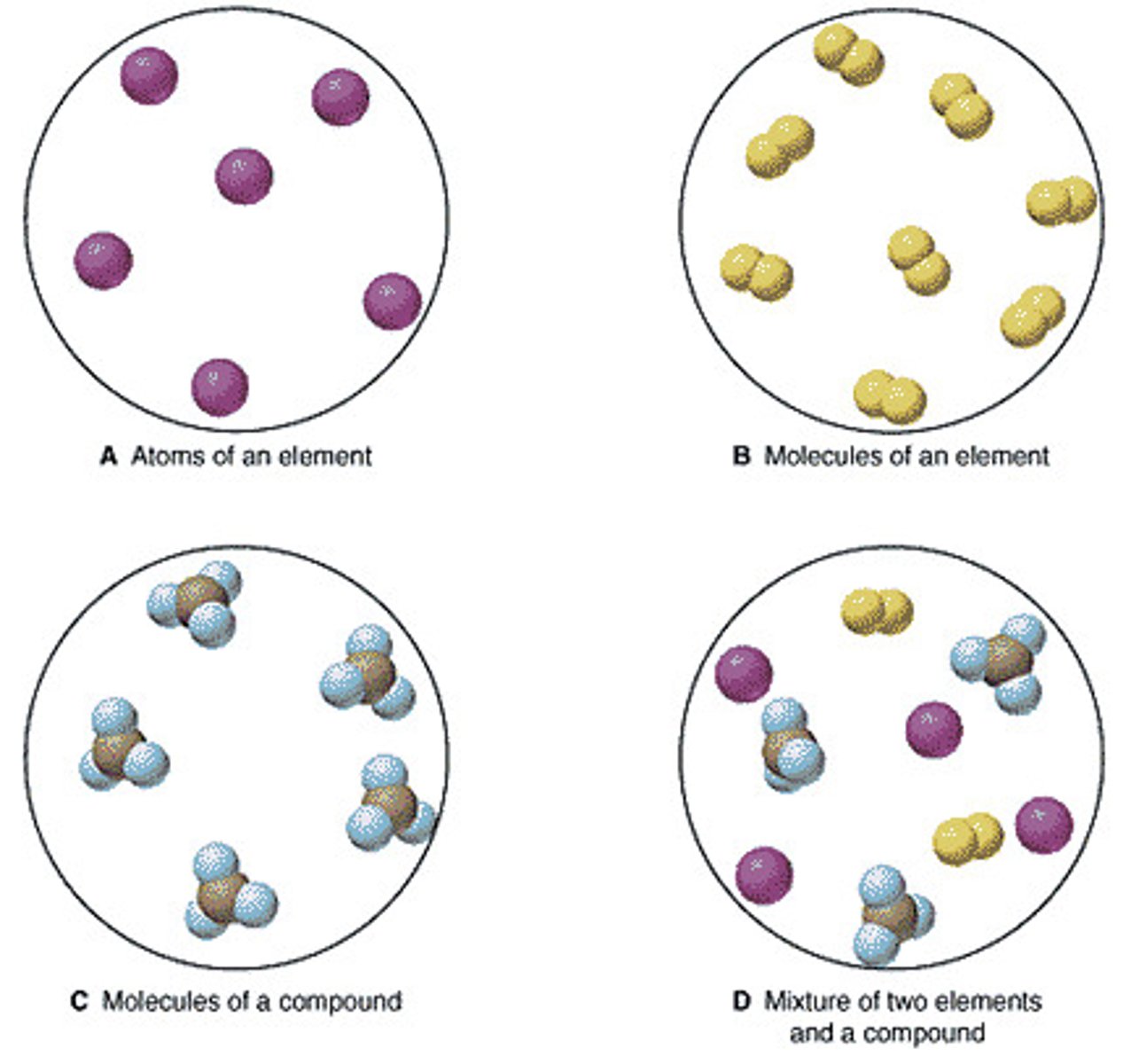
Compound
A substance that contains two or more different kinds of atoms chemically bonded together.
Condensation
Phase change from a gas to a liquid.
Deposition
Phase change from a gas to a solid. (skip liquid)
Element
A substance that can not be broken down into simpler substances. You can find it on the periodic table
Endothermic (adding heat)
When thermal energy (heat) is being added /
Think endo=INTO
solid to liquid (melting)
liquid to gas (vaporization)
Solid to gas (Sublimation)
Evaporation
The gradual vaporization of a liquid on the surface.
Exothermic (giving off heat)
When a substance is losing thermal energy (heat)
gas to liquid (condensation)
liquid to solid (freezing)
gas to liquid (deposition)
Freezing
Phase change from a liquid to a solid.
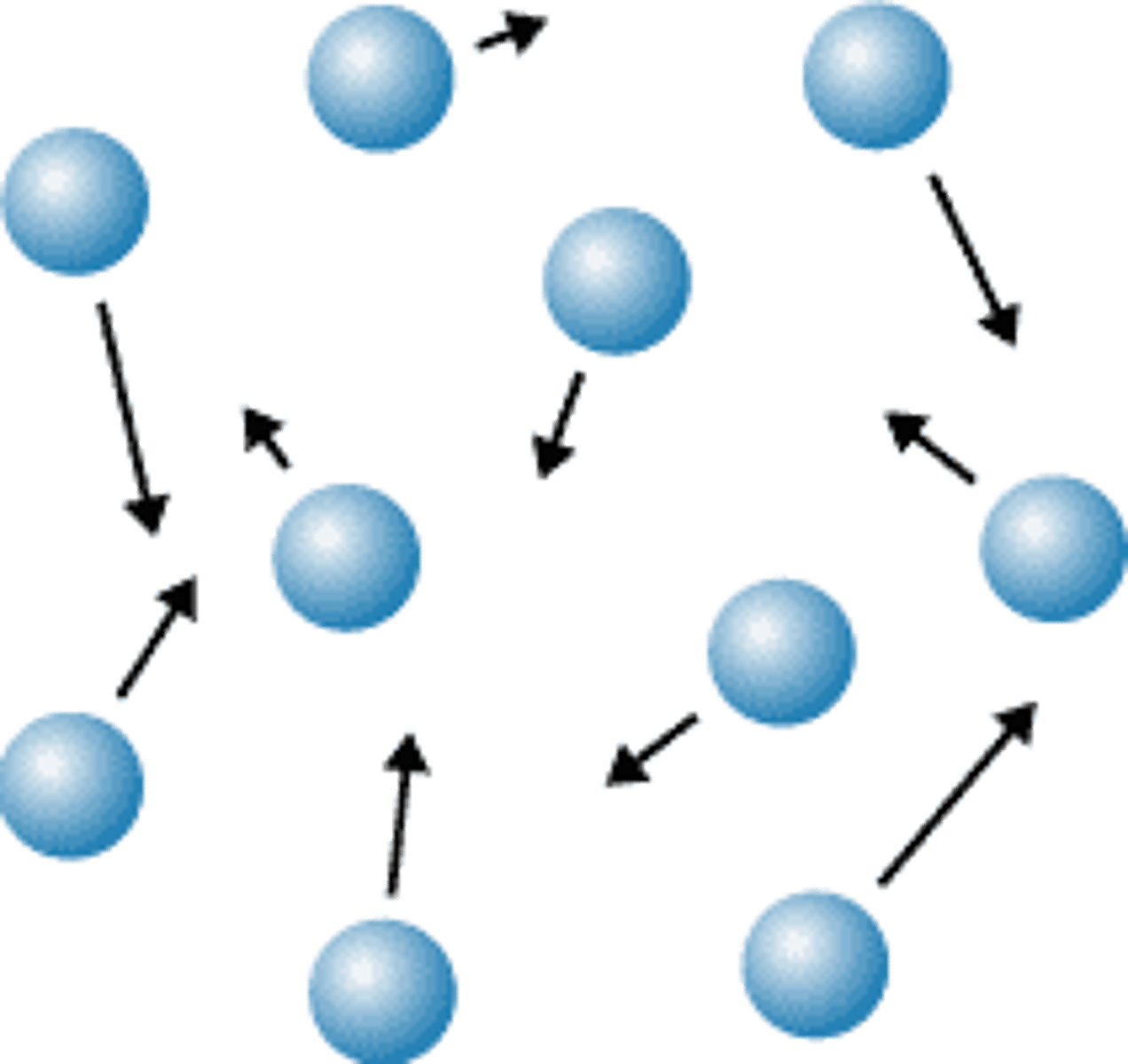
Gas
State of matter where all the atoms and molecules are independant of each other and move around randomly
Heterogeneous
When a substance looks like it is made of 2 or more things.
Chunk Salsa
Chocolate chip cookie
Homogeneous
When a substance looks like it is all the same throughout.
vanilla milkshake
peanut butter cookie
Liquid
State of matter where all the atoms and molecules are able to move around but they all stay grouped together.
Matter
Anything that has both volume (takes up space) and mass
Melting
Phase change from a solid to a liquid.
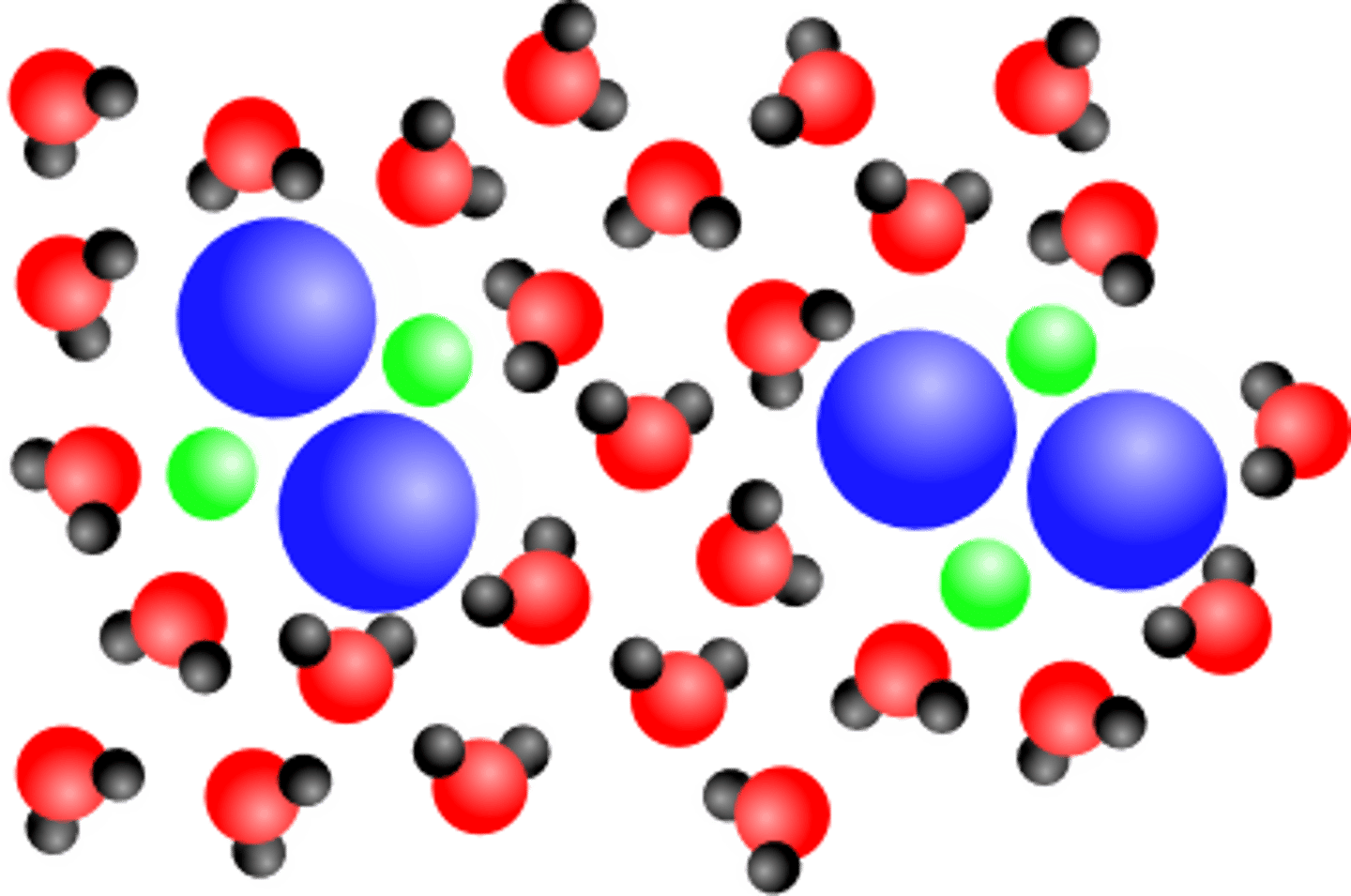
Mixture
Two or more substances combined that
CAN be separated
Molecule
Two or more atoms together.

Phase Change
Changing state of matter.
Plasma
State of matter that is higher energy than a gas.
Pure Substance
A substance that is made of only one type of atom (O or O₂) or one type of compound (CO₂).
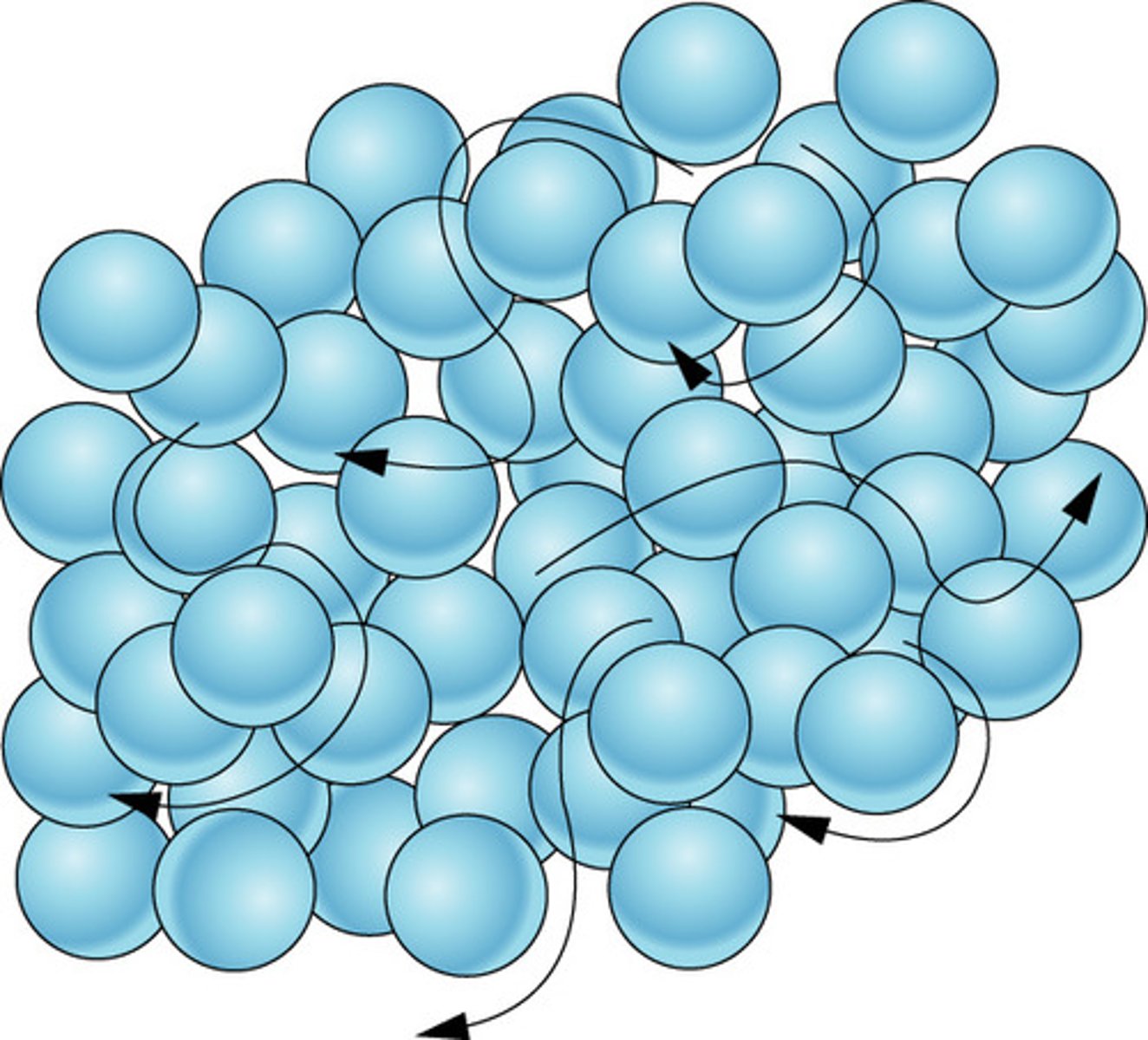
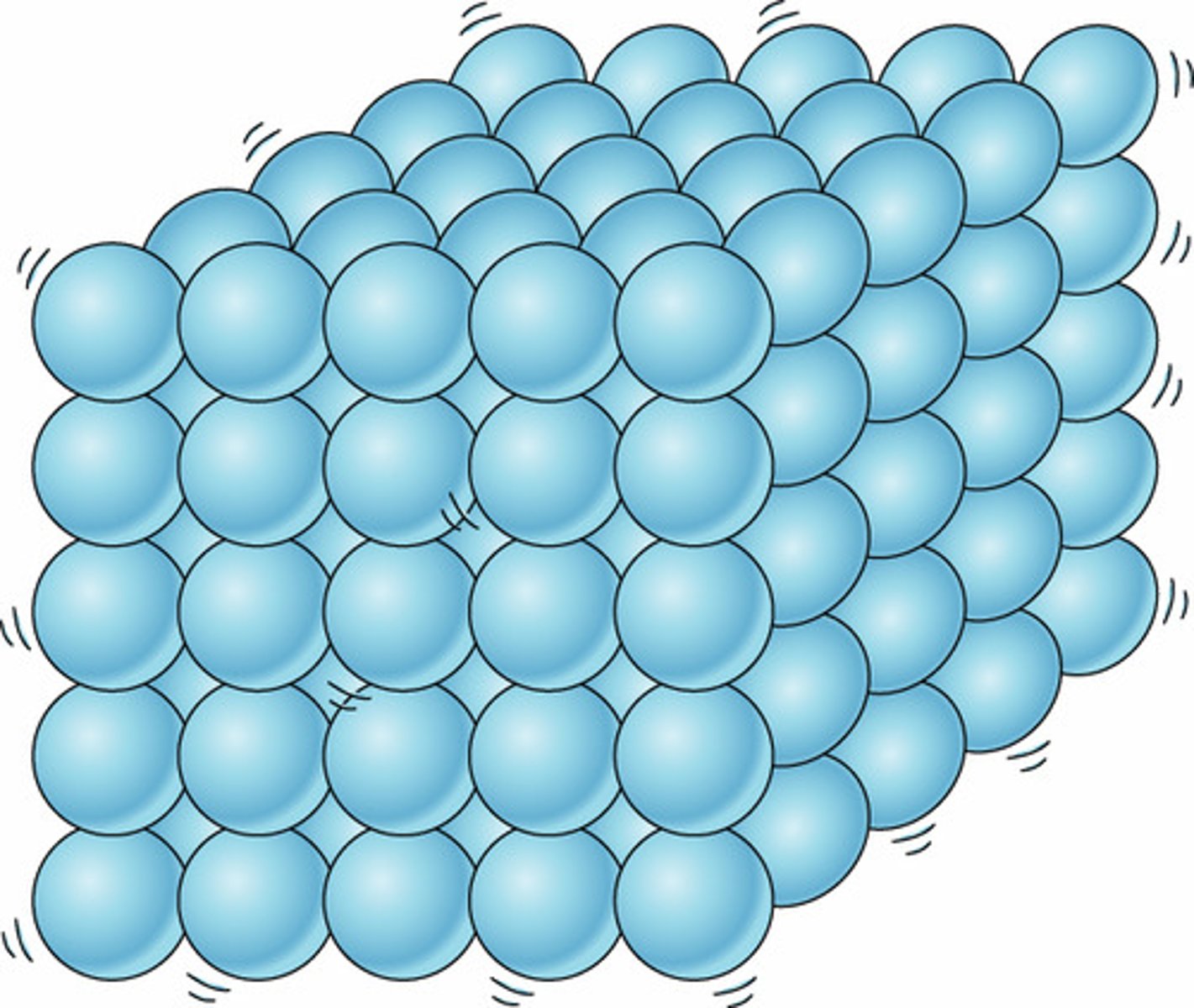
Solid
State of matter where all the atoms and molecules are arranged in a neatly ordered pattern.
Sublimation
Phase change from solid to a gas (skip liquid)
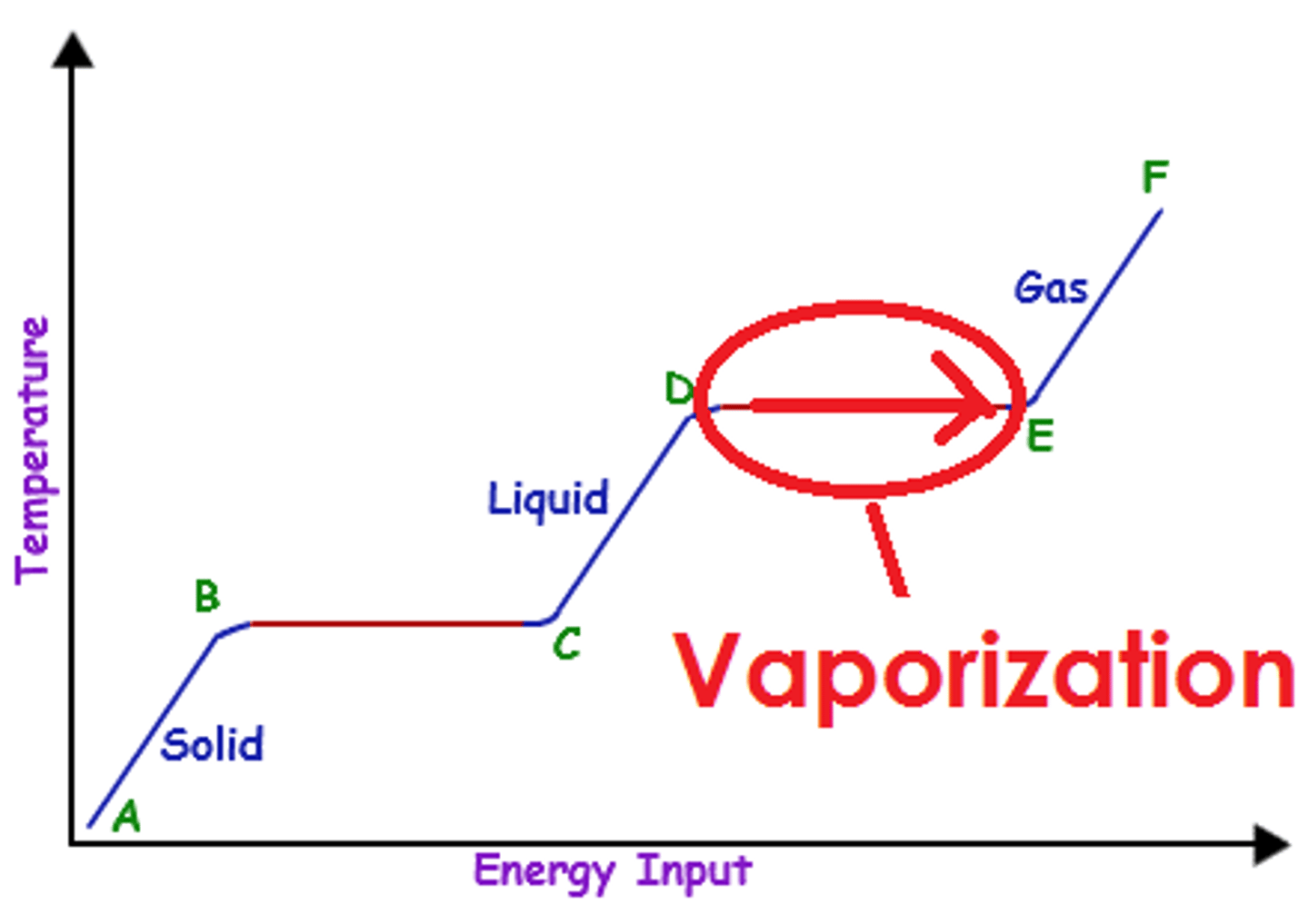
Vaporization
Phase change from liquid to gas which includes...
...boiling
...evaporation
Examples of Solutions
salt water, coffee (no creamer), sugar water, Kool-aid, Coke, vinegar
Examples of Elements
carbon (C), oxygen (O),
nitrogen (N), chlorine (Cl)
Examples of Compounds
Water (H₂O), Sugar, Salt, Baking Soda, Baking Powder
Examples of Mixtures
soapy water, shells in sand, dirty water
Examples of Homogeneous things
tomato soup, salt water, legs of your desk, post-it note, plain hummus
Examples of Heterogenous things
vegetable soup, mixed jar of candy, junk drawer, cookie dough ice cream, Snickers bar
Exothermic (giving away heat) ←
Going from Liquid to solid, gas to liquid or gas to solid causes the atoms to move less. The material LOSES energy.