4. Hybridoma and Antibodies
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Antibody
A large Y-shaped protein used by the immune system to identify the neutralize pathogens; foreign objects such as bacteria and viruses. They recognize and bind to antigens, which are unique molecules on the surface of pathogens.
Characteristcs of antibodies
Class of proteins called immunoglobins (lgs), produced by speficic types of white blood cells called b-cells, natural defense “homing devices” against foreign
substances. Highly specific: One antibody (Ab) binds to one antigen.
How many types of Immunoglobins
IgG, IgM, IgA, IgD, IgE; All have the same based unit structure of a “capital Y” configuration. Differ from each other in size, charge, carbohydrate content, and amino acid composition. IgG: Most predominant antibody
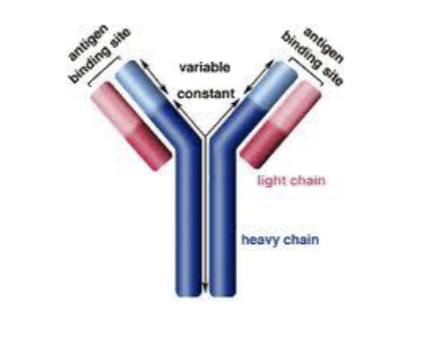
Overview of the “Y” Structure
Antibody basic structure consists of: 2 Heavy Chains, 2 Light Chains
Each chain consist of: Constant Domain(s), Variable Domain(s)
Putting it together: Each light chain has 1 variable and 1 constant domain
Antibodies have diverse structures in the “Variable Region”
Functions of Antibodies: Inactivation
Bind and block activity of toxins
Functions of Antibodies: Agglutination
Precipitate and isolate until other cells perform “cleanup”
Functions of Antibodies: Tagging of invaders
tag invader for processes such as phagocytosis, complement activation, or Killer T cell attack
Human Immune Response
Non-self recognition: Substances without specific “self” markers on major histocompatibility complex (MHC) are discerned and targeted for destruction. Once activated by the helper T cell, the B cell divides into plasma cells (shown at left) and memory cells (not shown). Memory cells remain in the body to help the immune system activate much more quickly if the antigen appears again.
Antibody Properties: Affinity
Measure of binding strength for a single bond
Antibody Properties: Avidity
The combined strength of multiple bond interactions
Antibodies Many Purposes
Cell classification by flow cytometry
• Protein quantitation using ELISA
Immunoassay
• Immunohistochemistry
• Cellular probes
(Immunofluorescence microscopy)
how can we make antibodies?
Purification from animal serum by injecting a known antigen into an animal, which will then produce polyclonal antibodies that are . . .
– Not highly pure (mixture multiple molecules)
– Able to “cross react” with other antigens
– Made from many B-cell clones
– Produced only as long as the animal lives
Monoclonal Antibodies (mAbs) are homogeneous populations of
molecules that ...
Are made from a single B cell clone
• Bind to a single epitope of a single antigen
• Are all in a single antibody class
• Have the same antigen binding site
• Have a much lower potential of cross-reactivity
• Are more manufacturable
• Are more highly characterized and more “pure”
• Have no issues with long-term supply
So how can we make mAbs?
Mouse is injected with antigen and
produces antibodies.
• Mouse is sacrificed; antibody producing B-
cells from spleen are isolated.
• These B-cells are fused with immortal
myeloma cells, typically from human bone
marrow—forming hybridomas.
• Hybridomas are cultured in a specific
medium containing hypoxantine,
aminopterin, and thymidine (HAT); unfused
cells die.
• Hybridomas that produce antibodies
binding to original antigen are selected and
isolated.
• These hybridomas are grown in animals or
cultured in large-scale reactor vessels.
• Monoclonal antibodies are produced by
those cells and can be purified
Recombinant DNA Technology
Introduces the ability to combine hybridoma genetic information with human antibody genetic information, Reduces possibility of production of human anti-mouse antibodies in a patient by “humanizing” the molecule (partially or completely), Enables recombinant DNA to be
introduced into CHO cells to manufacture humanized antibodies