Solutions Y7
1/12
Earn XP
Description and Tags
Study notes
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Define Soluble and Insoluble
Soluble substances can dissolve in a solvent. Insoluble substances cannot dissolve in a solvent.
Is flower soluble or insoluble in water?
Insoluble
True or False: A solution with a substance dissolved in it weighs less the a solution and a undissolved substance.
False. They would weigh equally due to conservation of mass.
What is the difference between a solute and a solvent?
A solute is the substance that is dissolved in a solution, while a solvent is the substance that dissolves the solute.
Step by step of how you find if one of two beakers of water contain salt.
Set both beakers over a Bunsen Burner, on top of a tripod and gauze.
Light the Bunsen Burner
Turn off the Bunsen Burner once the majority of the solution has evaporated from both beakers.
Leave the Beakers in a warm, dry place for a week
Observe the results
(One Beaker should have a collection of obvious salt crystals)
How can you tell if a solute is still present in a solution, even if it cannot be seen?
You could boil the solution.
Define solubility
Solubility is the ability of a solute to dissolve in a solvent.
Define Saturated.
A saturated solution is one in which no more solute can dissolve in the solvent at a given temperature.
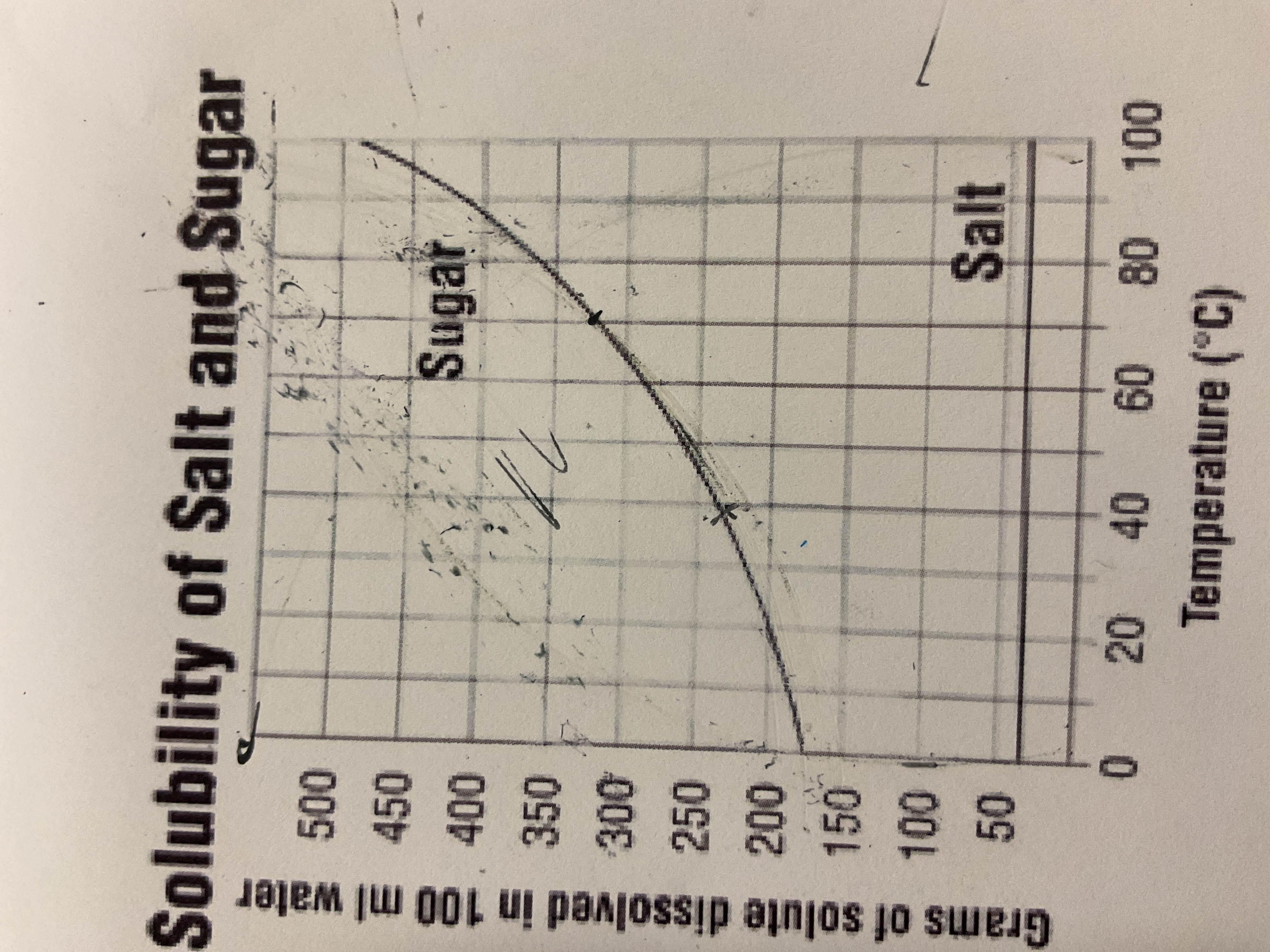
What is the mass of sugar at 90deggrees
410deggrees
How do you extract salt from rock salt?
Crush up the rock salt using a Mortar and Pestle .
Place the crushed up rock salt into a Beaker and add Water
Stir the mixture with a Glass Stiring Rod to help it dissolve.
Filtre the mixture with Filtre Paper placed inside of a Conicla Flask.
Heat the filtered solution over a Bunsen Burner (place the mixture in an evaporating basin before hand) until the majority of the solution has dissolved and salt crystals have begun to form.
Leave in a warm, dry place for a week.
Observe Results
True or false: A substance can be soluble or insoluble depending on the solvent.
True
Name a number of liquids that can be used as solvents.
Water, Ethanol, Acetone etc.
Give an example of how not all substances will dissolve in specific substances.
Water and Nail polish.