Lab J - Preparation of Bixin Extract from Annatto Seeds and its Thin Layer Chromatography
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
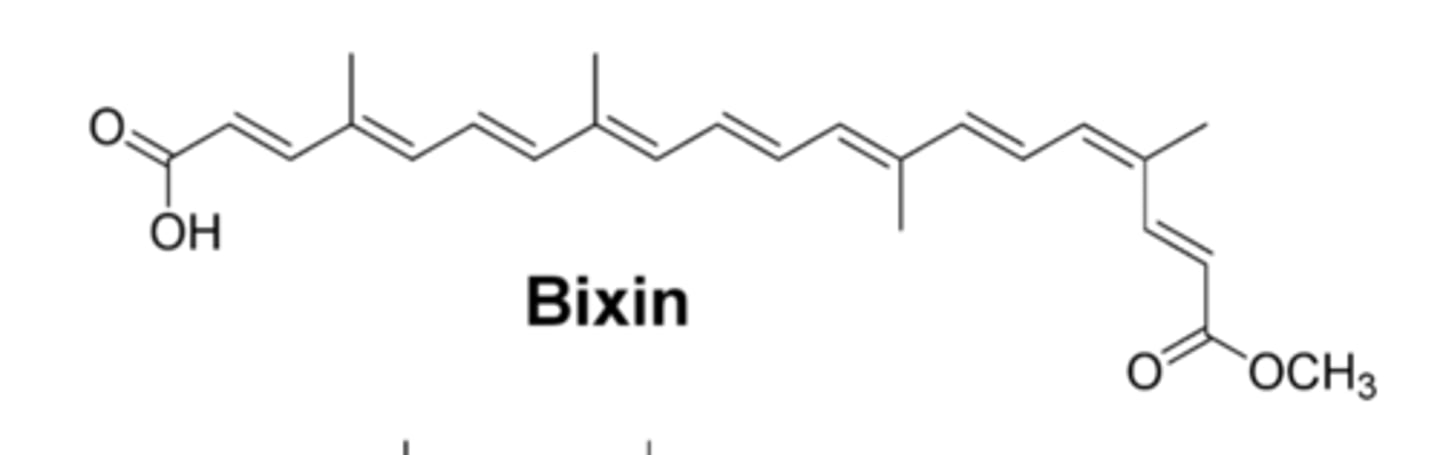
What is bixin?
a natural product isolated from the seeds of Bixa Orellana, aka annatto tree.
What is a carotenoid?
Naturally occurring pigment. Annatto extract consists of a number of different types
Absorption at UV and visible wavelengths
promotion of e- from HOMO to LUMO
beta-carotene absorbs at what wavelength
wavelengths of 453 and 483 nm (blue light) which leads to its orange color
lycopene absorbs at what wavelength?
505 nm in the green region
- this leads to its red color
What does extended conjugation do?
Reduces HOMO->LUMO gap (lower E), which lowers the frequency (E=hv), leads to absorption at longer wavelengths (v=c/lambda).
Increasing conjugation -> increase wavelength of absorption
observed color is ________
complementary to the color of the wavelength absorbed
why do we not wet the filter paper during vacuum filtration?
if you wet it, you will end up w/ the formation of two phases (phase separation)
organic phase --> containing 10% ethanol
aqueous phase --> containing water
Why do we use multiple extractions of the annatto seeds instead of just one?
it improves/gives you a better theoretical recovery
What is chromatography?
qualitative analytical tool. separates components in a mixture, helps in following progress of chemical reaction, no restriction on sample type (organic, inorganic, biological, medial), and high sensitivity (detection of 10^-6 g or less)
Two phases in chromatography
stationary and mobile phases (SP and MP)
What does SP do?
stays as is
What does MP do?
flows around SP
What is physical form of SP?
fine solid with lots of surface area
What is physical form of MP?
fluid (liquid or gas)
How does SP affect sample movement?
retains sample by surface interaction (based on polarity of sample)
How does MP affect sample movement?
helps move sample along
What did we use for SP in this lab?
thin layer (250 microns) of silica gel (SiO2)
Types of chromatographic methods
thin layer chromatography (TLC), liquid chromatography (LC), high performance LC (HPLC), and gas chromatography (GC)
Silica gel structure
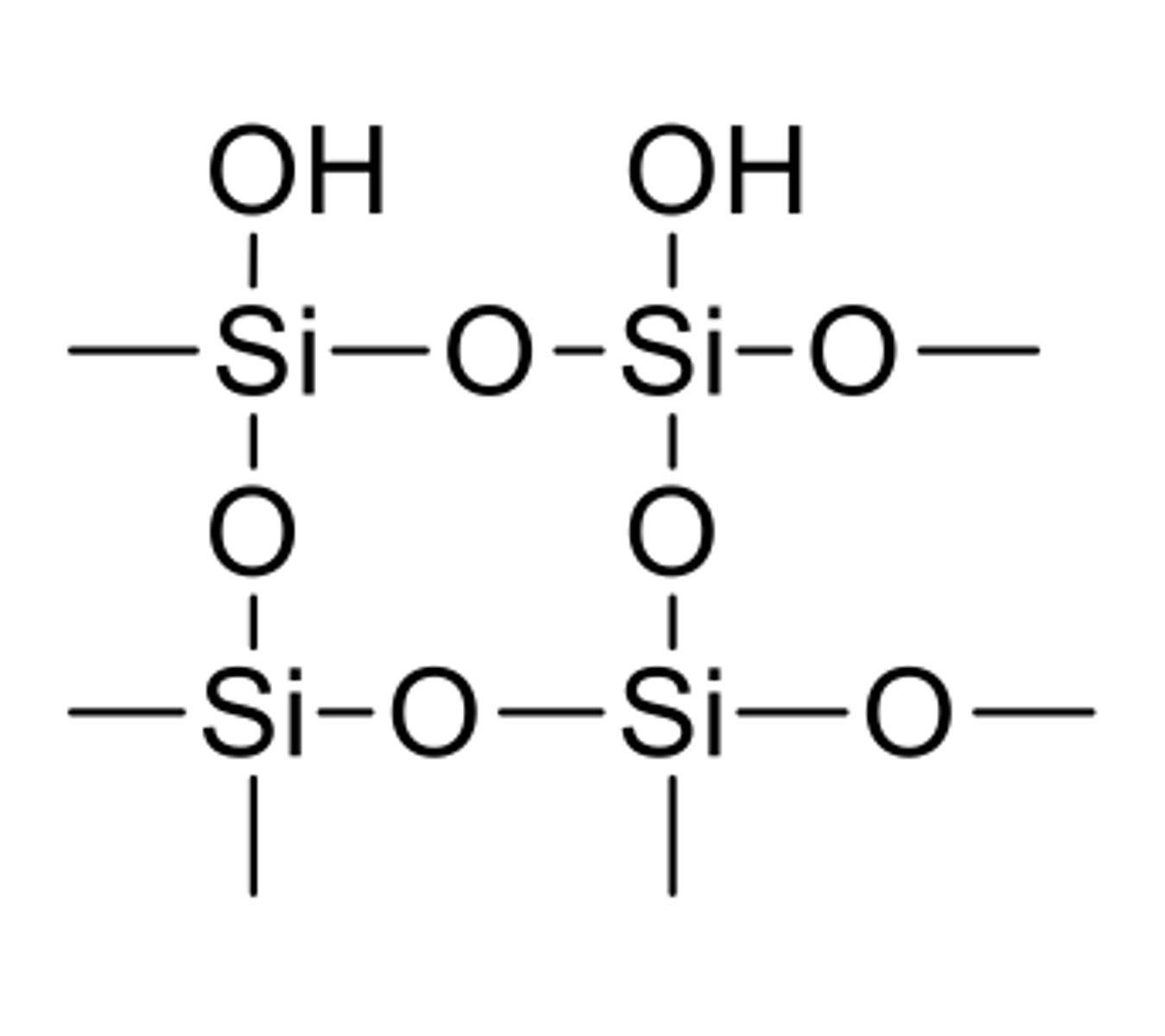
Silica gel stats
- most commonly used, inexpensive SP.
- Silonal (Si-OH) group are very polar, thus creating a very polar surface
- can be impregnated with a fluorescent indicator and put on a plastic backing.
- polar molecules stay and move less (smaller Rf), nonpolar or less polar molecules move more (larger Rf)
Rf meaning
ratio to the front, ratio of distances, Retention Factor
Rf equation
Distance traveled by substance / Distance traveled by solvent front
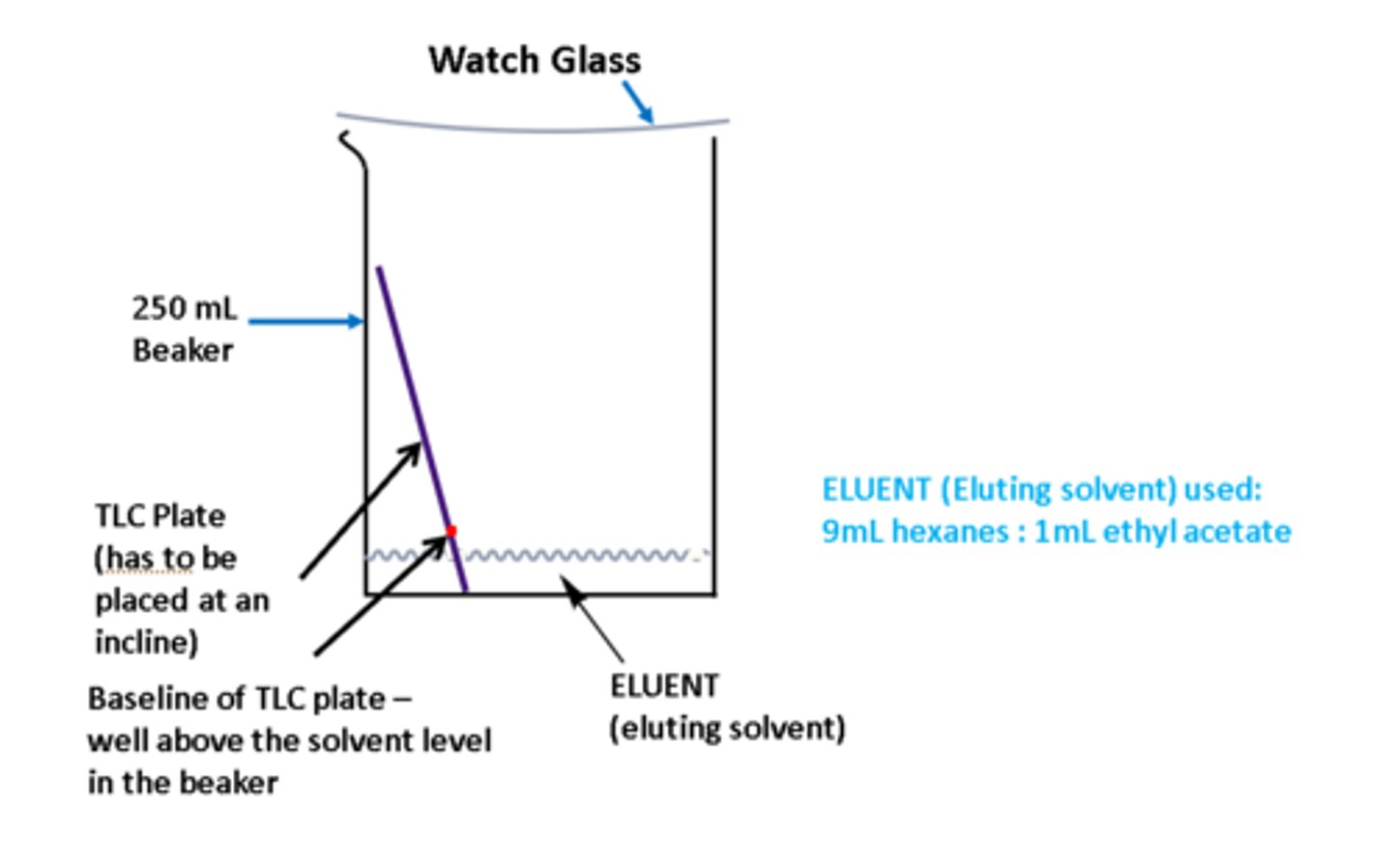
Steps for TLC
1. application of sample using TLC capillary tube (open on both ends), spot lightly and mark with pencil. use rough side
2. development of sample in TLC development chamber
3. visualization of sample - can be under UV light
4. interpretation of results (compare Rfs)
TLC development chamber scheme
Bixin structure
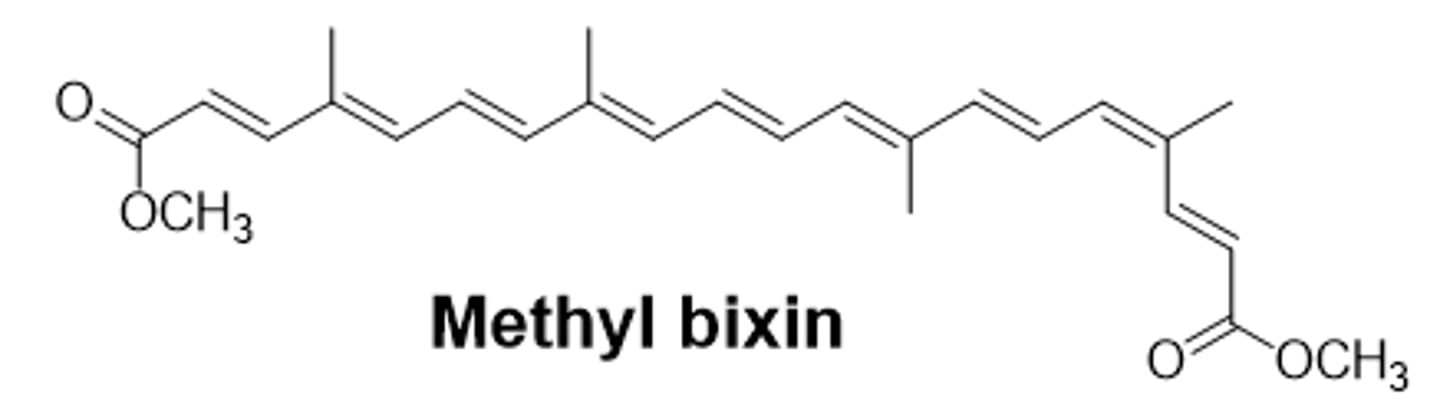
Methyl bixin structure
Norbixin structure
Methyl bixin will have ______ Rf values
larger Rf values because its the least polar
- no affinity and won't bind to the silica gel so it travels further
- most nonpolar compared to bixin and norbixin
more polar compounds will lead to ______ Rf values and ______ distance traveled on the TLC plate
smaller / shorter
less polar compounds will lead to ______ Rf values and _________ distance traveled on the TLC plate
larger / longer
What lanes were on our TLC plates
Lane 1: one spot of our annatto extract
Lane 2: two spots of our annatto extract
Lane 3: one spot of bixin standard spotted by TA
Safety precautions for Lab D
- DCM is a carcinogenic compound. Always keep covered an inside fume hood
- Double glove
- If gloves damaged/soiled/contaminated, remove gloves, wash hands 20 sec w soap and warm water, and replace w new pairs of gloves
Waste disposal for lab D
- annatto seeds + filter paper in biohazard waste box
- rinse glassware w acetone and empty into acetone rinsings
- used capillary tubes in sharps
- solvents in halogenated waste
How many double bonds in conjugation do bixin and its derivatives have?
11
How can bixin, methyl bixin, and norbixin be separated?
they have different polarities due to different functional groups (methyl ester vs carboxylic acid)
What is the purpose of using different solvents for our TLC?
Determine best solvent for separation of the components, in which bixin moves sufficiently off the baseline so you can isolate with column chromatography
Optimal Rf for bixin
0.25-0.5
What did we use for MP/solvents in this lab?
DCM (most nonpolar), 3% EtOH in DCM, and 10% EtOH in DCM (most polar)
Which solvent did we determine was the best for separating bixin?
3% EtOH in DCM
What does increased solvent polarity do?
Increases Rf for all compounds on TLC plate
Dichloromethane is a very ______
volatile (don't want it to evaporate)
Why don't you need to put the TLC plates in a UV chamber?
they are highly colored and visible
What do you observe from the DCM TLC plate?
- DCM is relatively nonpolar
- only component that moves up the plate is methyl bixin (has 2 ester end units)
- NO SEPARATION of bixin run in plain DCM
What do you observe from the 10% EtOH in DCM?
- bixin traveled much larger distance because the solvent polarity has been increased; close to the solvent front
- leading edge of methyl bixin so there's a mix of methyl bixin and bixin
- no well defined separation between methyl bixin and bixin
- has higher Rf value, but is still mixed in and too close to methyl bixin to give us any optimal separation
what do you observe from the 3% EtOH in DCM?
- bixin is away from methyl bixin --> clearly separated
- solvent system w/ best separation
influence of solvent polarity on separation of bixin
as u increase polarity of the solvent by adding ethanol to DCM, the spotted compounds traveled larger distances and have larger Rf values
3% ethanol in DCM
What's the point of using the rotary vap?
to remove the 10% ethanol in DCM solvent to carry out the forward extractions
- what we will have left is the bixin extract
if the Rf of bixin is too high, the compound will move off the column very quickly which will not allow separation to occur
If the Rf is very low, it will be difficult to get the compound to _____ of the column
elute
In the optimal separation, the bixin should move off the baseline and show significant distance from all other compounds. What should the value be?
0.25-0.5