Science 10- Unit A: Sec 3 Review
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
what is a chemical reaction
a chemical reaction is a rearrangement of atoms
what is chemical change a result of
chemical change is a result of a chemical reaction where new substances are formed
How does a chemical reaction happen (remember bonds)
the bonds of each reactant are broken, the atoms are rearranged to form new bonds creating product

list characteristics of chemical reactions
1. colour change 2. formation of a gas 3. formation of a precipitate 4. energy change (exothermic and endothermic) 5. change in odour

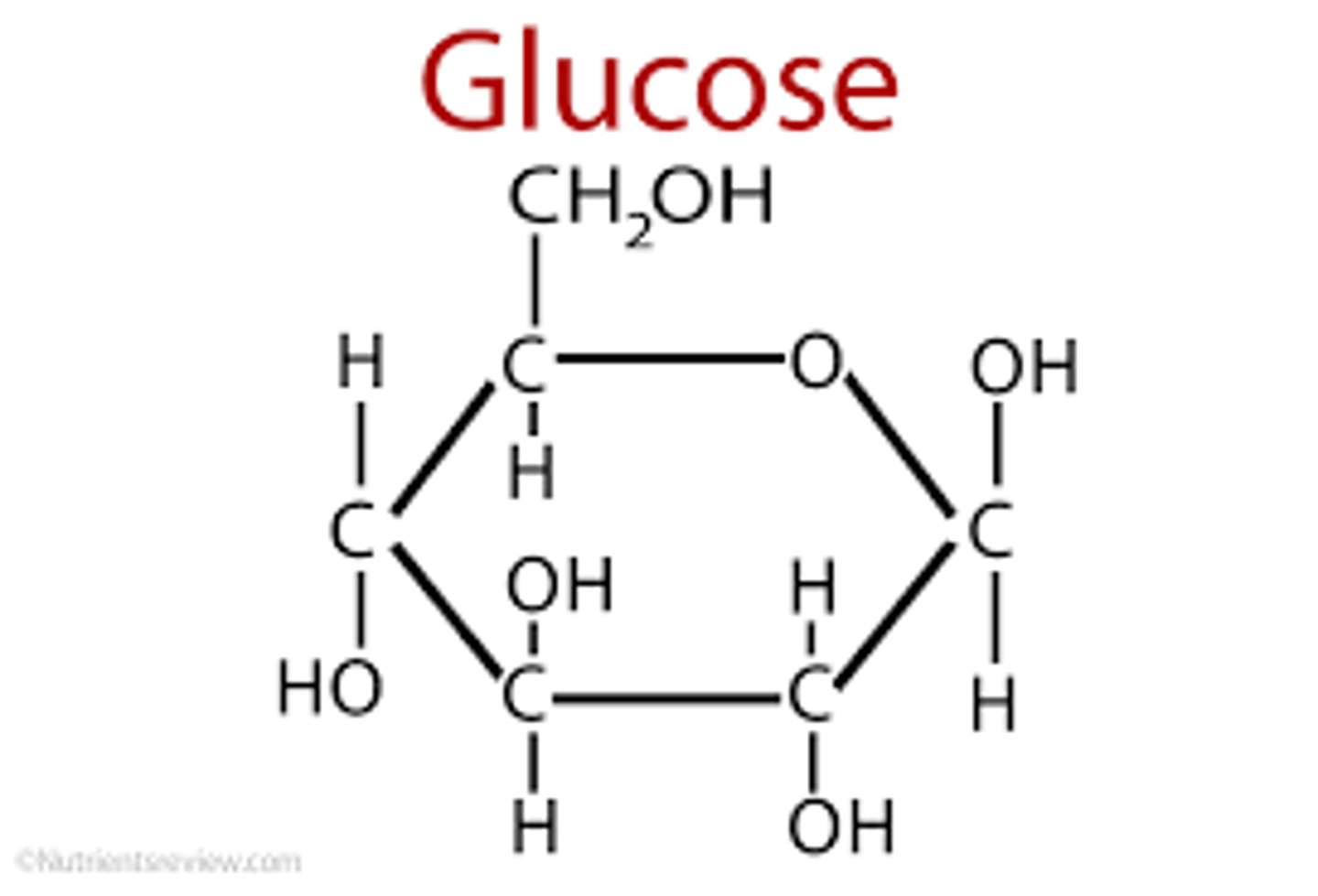
what is the formula for glucose
C6H1206
what is the formula for ammonia
NH3 (g)
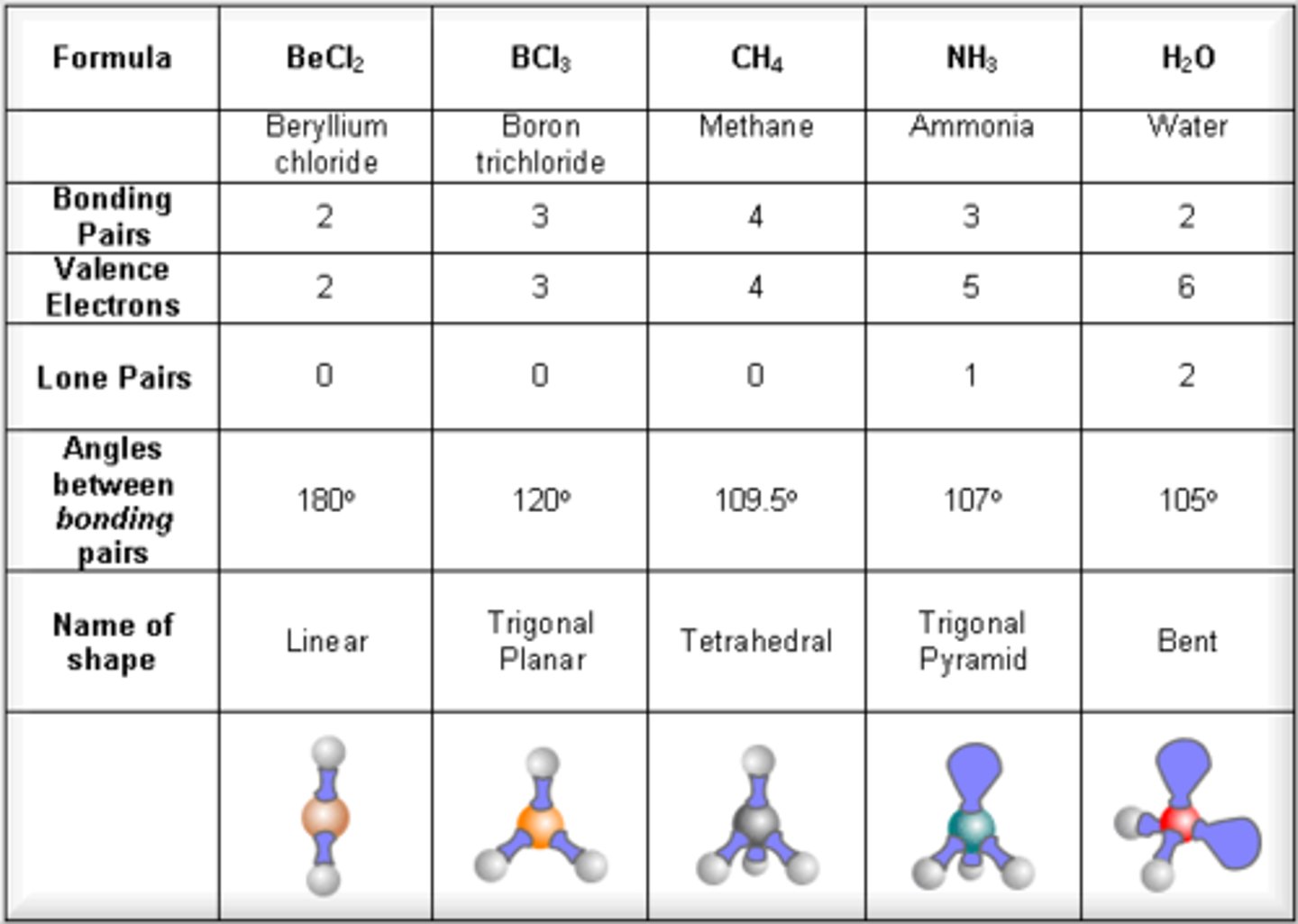
what is the formula for methane
CH4 (g)
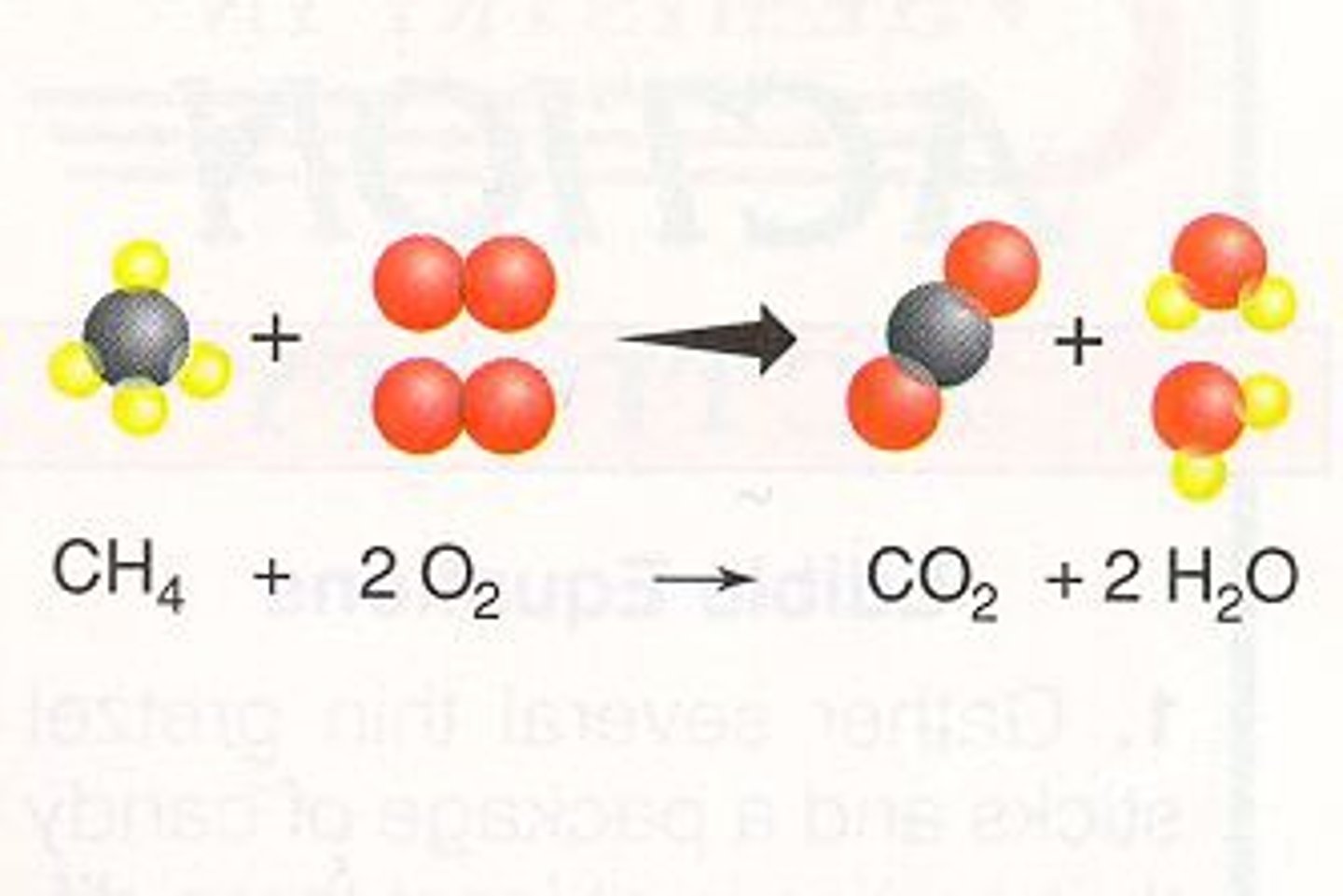
What type of reaction is photosynthesis in terms of energy
endothermic, absorbs energy from the sun
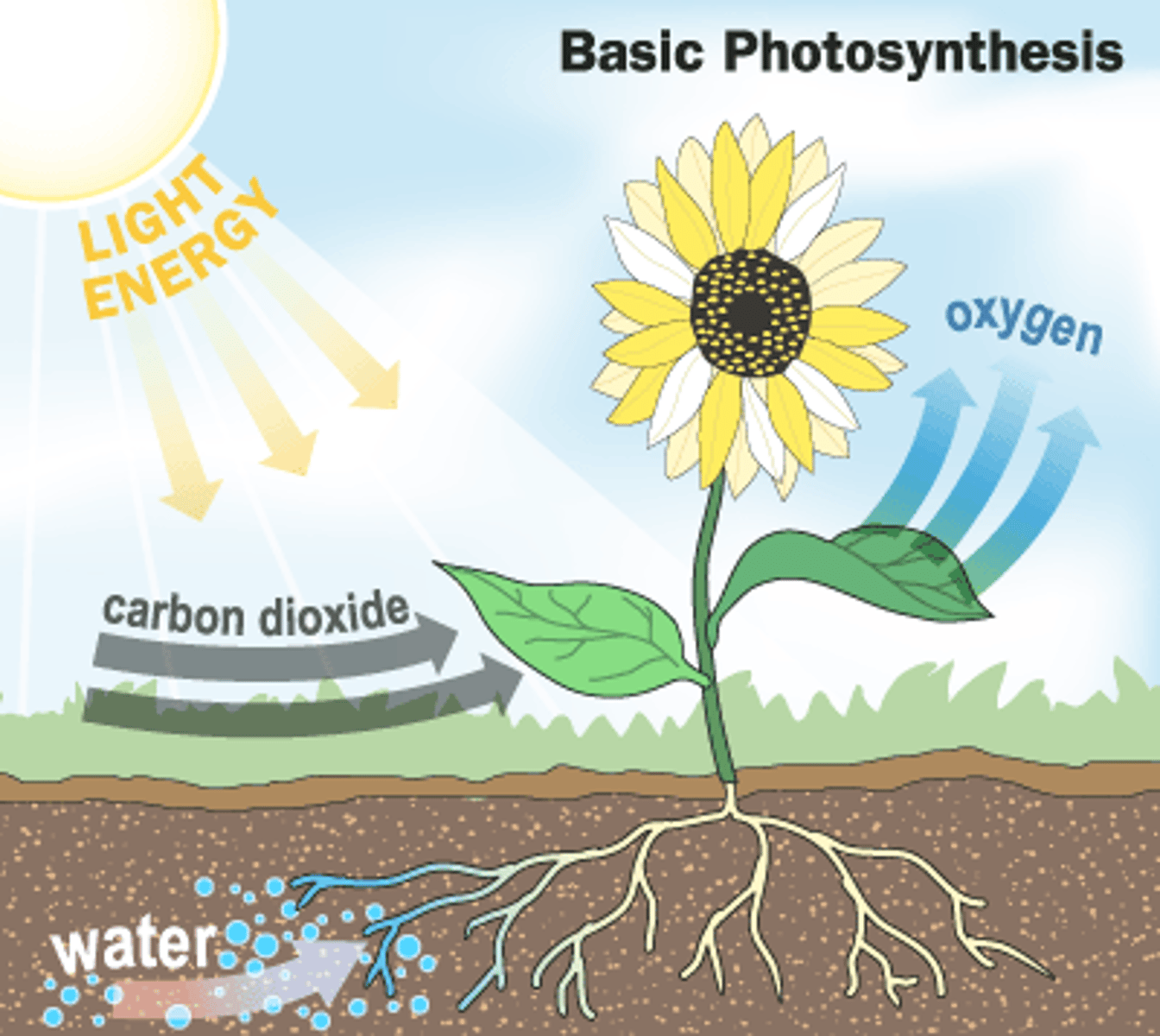
what is the formula for photosynthesis
6CO2 + 6H2O ------> C6H12O6 (glucose) + 6O2

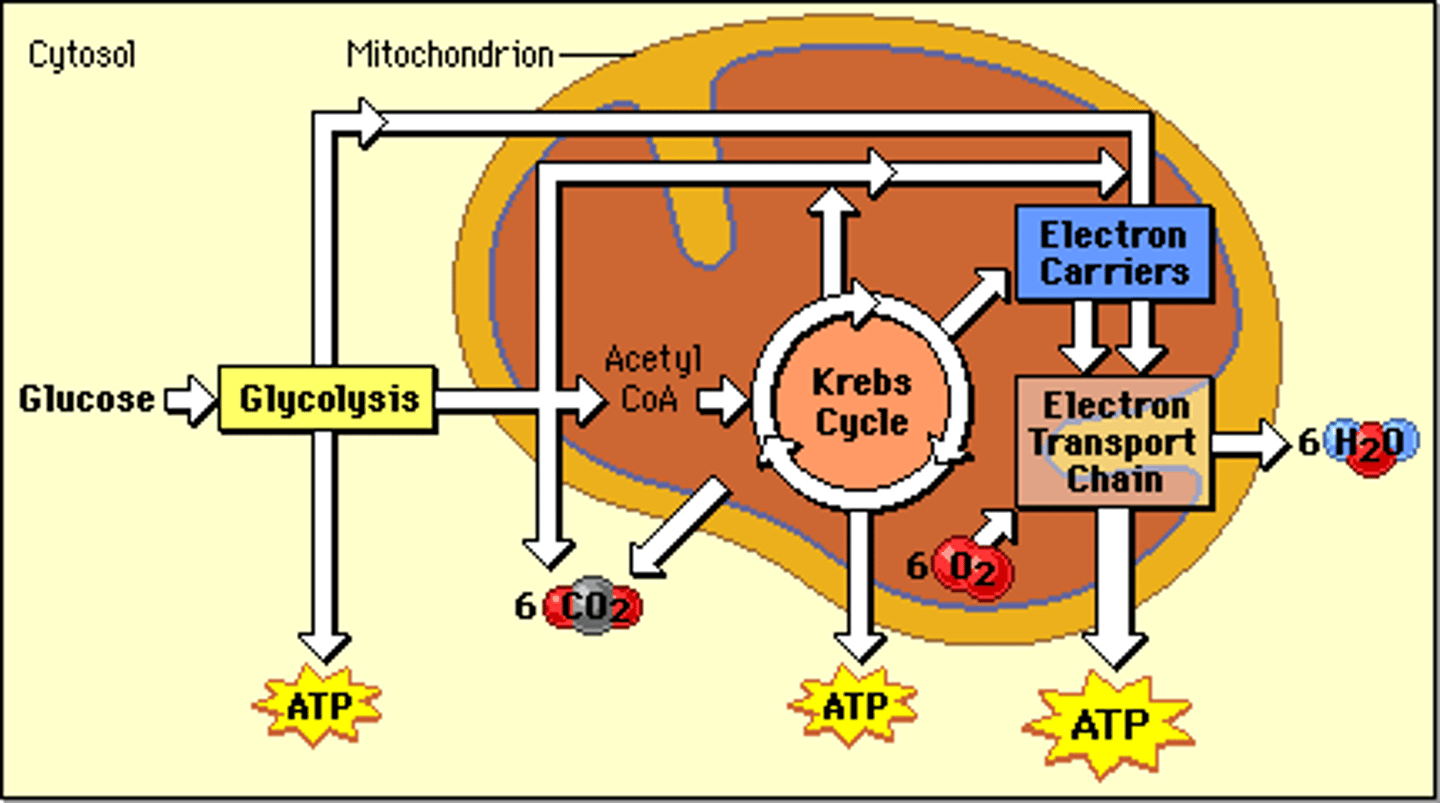
what type of reaction is cellular respiration in terms of energy
exothermic, releases thermal energy
What is the relationship between photosynthesis and cellular respiration?
they are reverse processes of one another
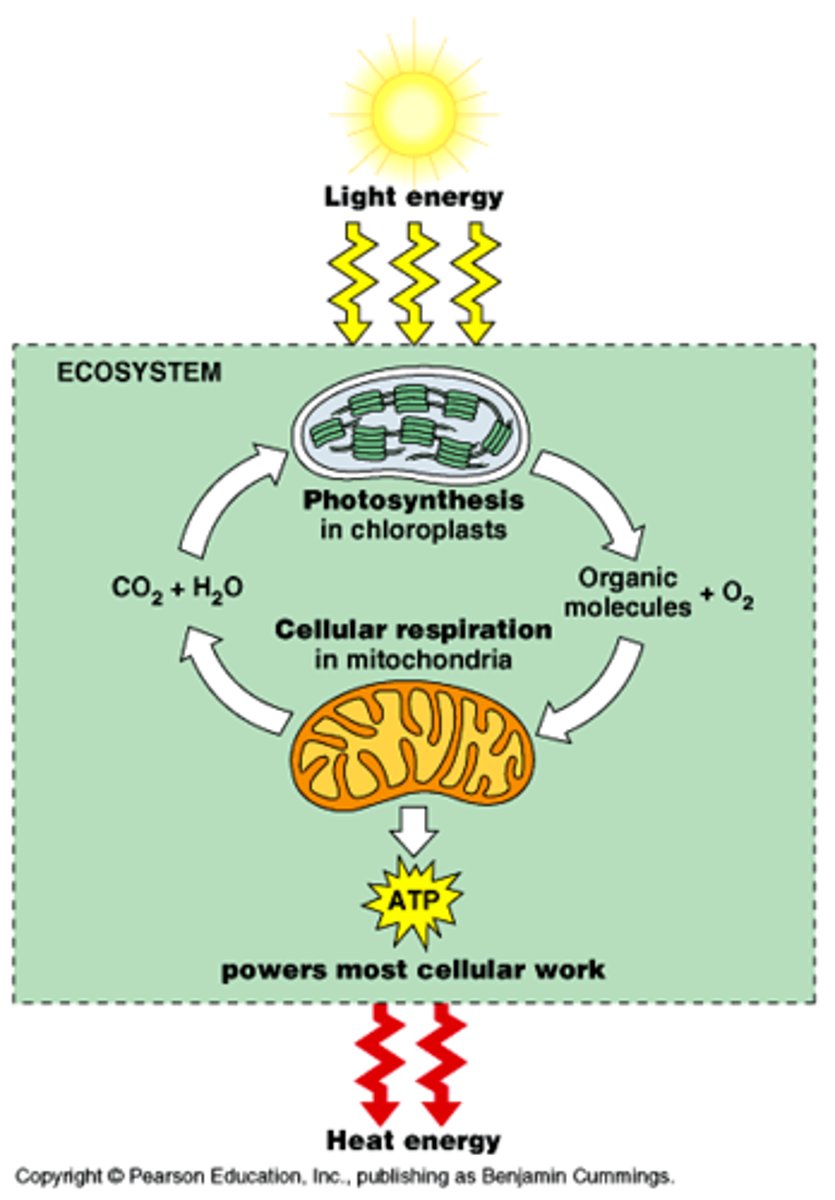
what is the formula for cellular respiration
C6H12O6 + 6O2 --> 6CO2 + 6H2O
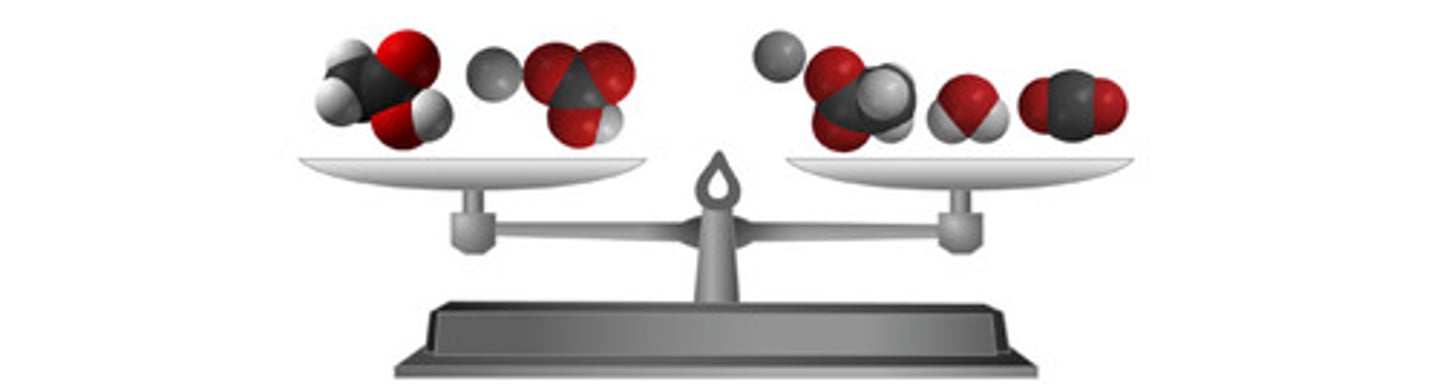
what is the law of conservation of mass
the law of conservation of mass states that mass must be conserved, total number of atoms in reactants must be equal to the total number of of atoms in the products.

what happens to atoms of the reactants
they are simply rearranged to form new products. bonds between atoms are broken to make products without the loss of atoms.
what are the five different types of chemical reactions
1. formation 2. decomposition 3. single replacement 4. double replacement 5. hydrocarbon combustion
what is a formation/synthesis reaction
a reaction in which elements combine to form a single compound. Ex: formation of carbon monoxide
2C+02>2CO

what is a decomposition reaction
reverse of a formation/synthesis reaction. a compound is separated into its constituent elements
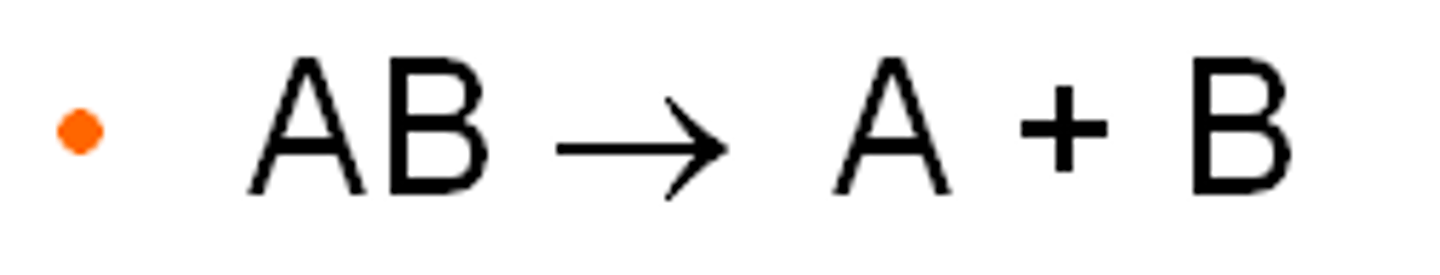
what is a single replacement reaction
a single element replaces an element in the compound. always takes place in solutions (aq) . Ex: 3 Cl2 (g)+ 2 NiBr3 (aq) >> 2 NiCl3 (aq) + 3 Br2 (l)
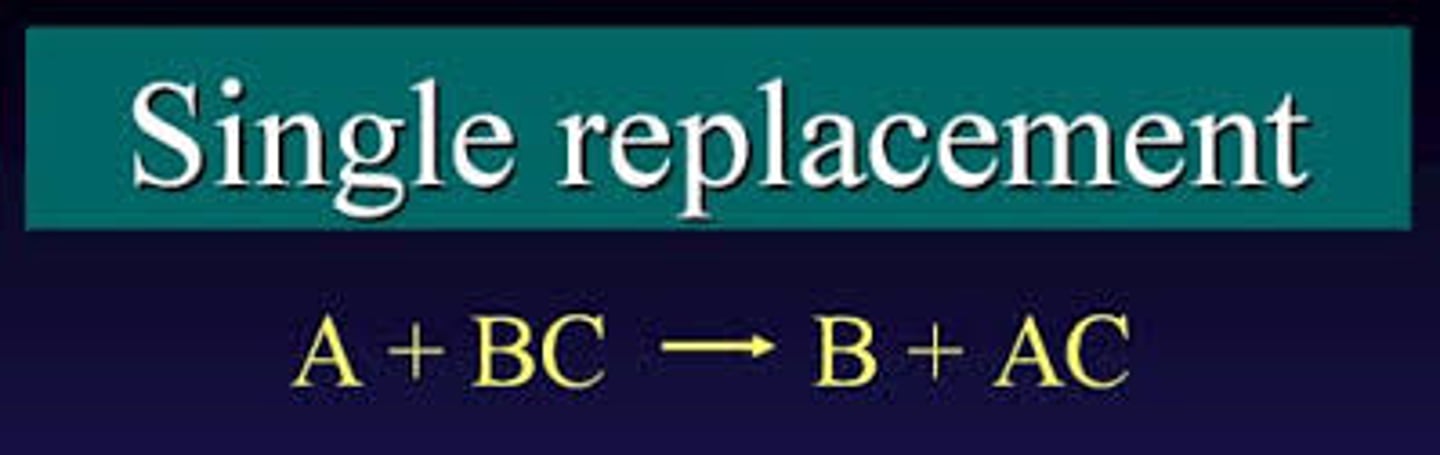
what is a double replacement reaction
two ionic compounds react. the metals and non metals switch "partners", which often forms a precipitate. Ex: Pb(NO3) 2 (aq) + K2Cr2O7 (aq) >> 2 KNO3 (aq) + PbCr2O7 (s)
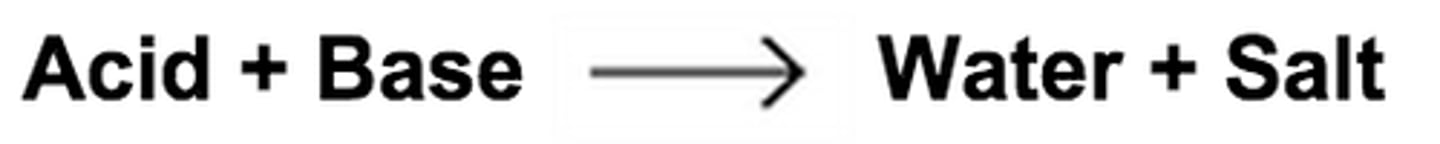
what is a hydrocarbon reaction
a hydrocarbon always reacts w oxygen and the products are always carbon dioxide+water vapor. Ex: 2 C4H10 (g) + O2 >> 8 CO2 (g) + 10 H2O (g).
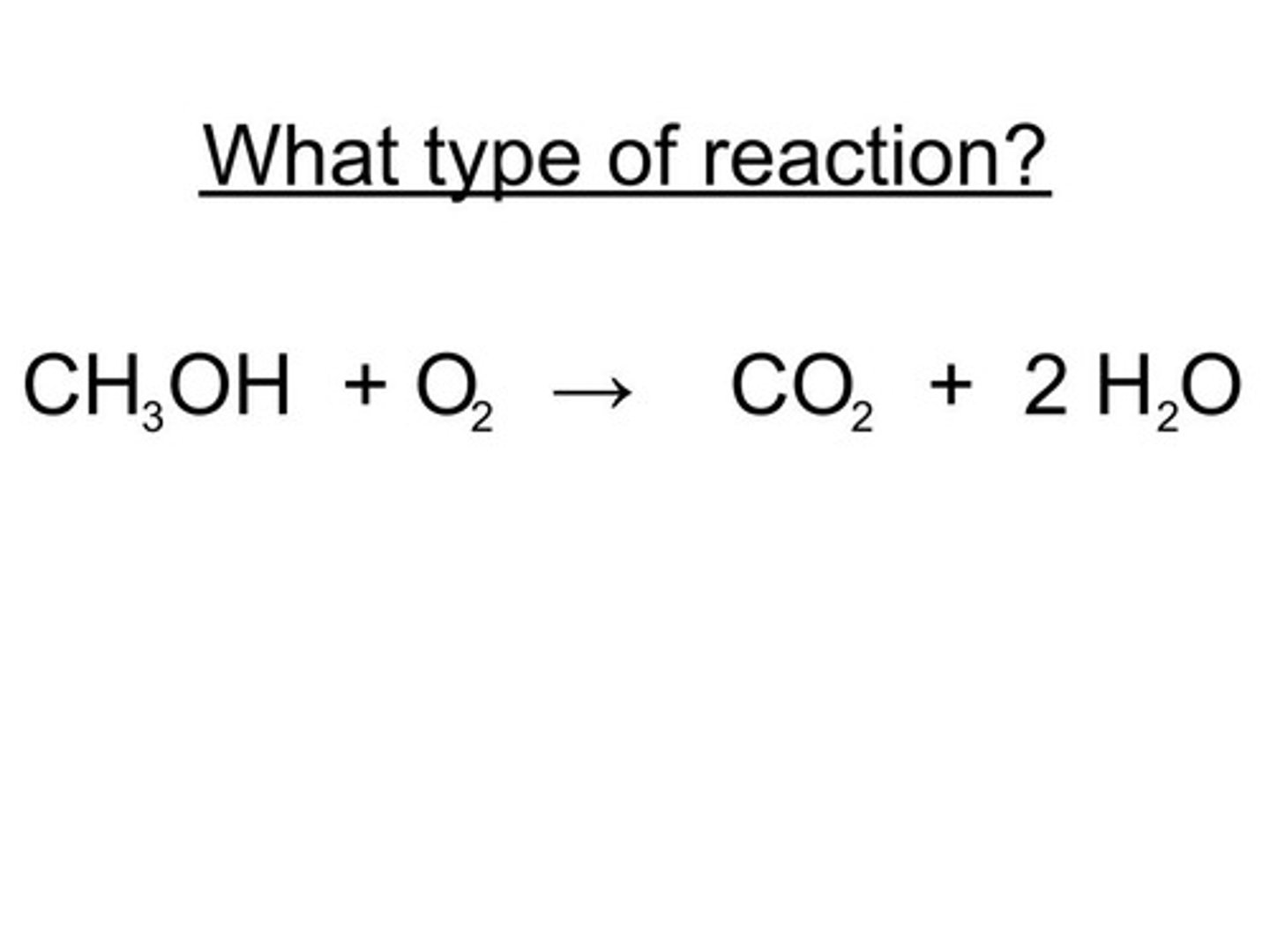
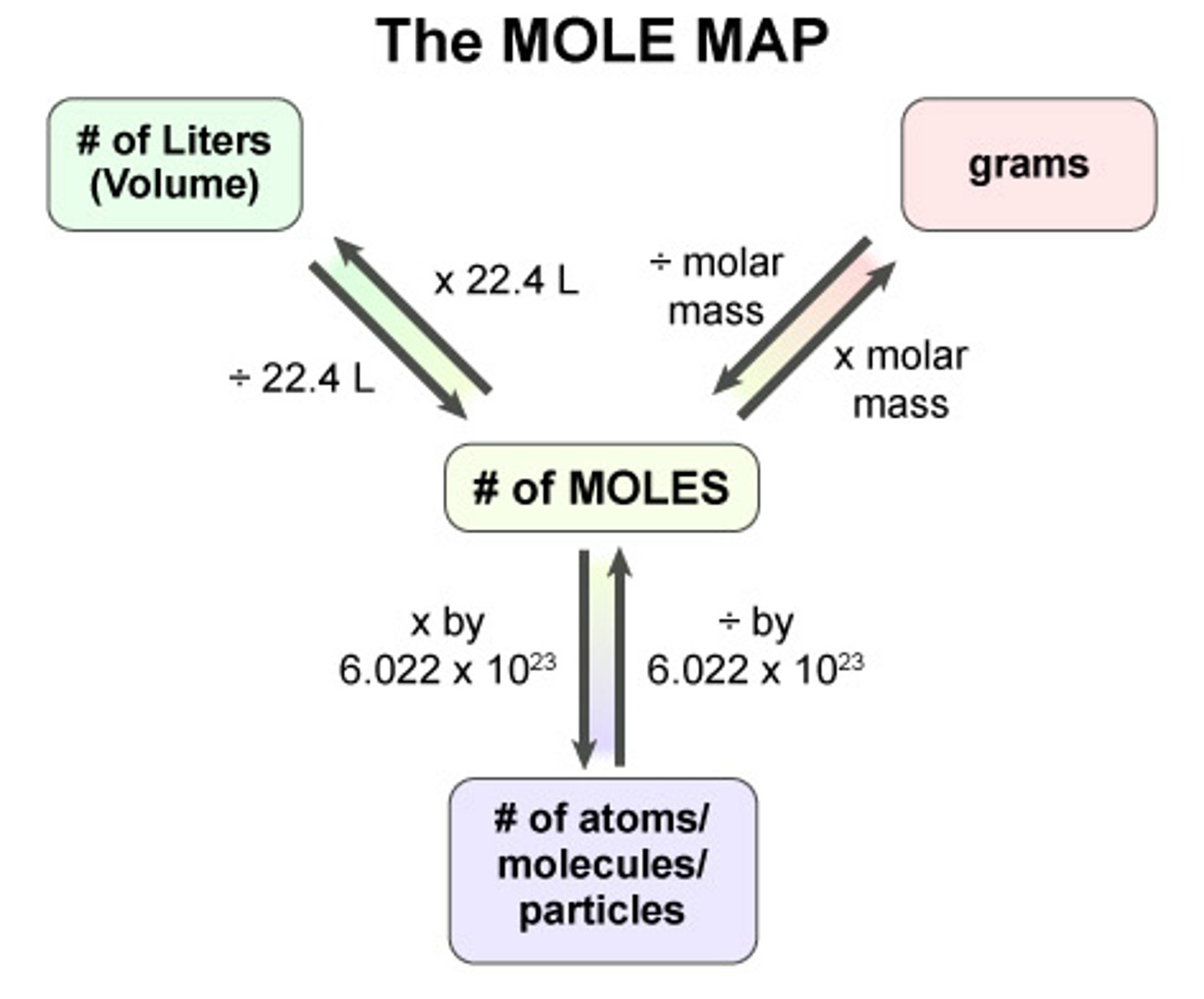
what is a mole used to describe and what is it a unit for
a number quantity. the mole is an SI unit for the chemical amount in a substance

how big is a mole
6.02x 10^23

what is the mole referred to as?
avogadro's number
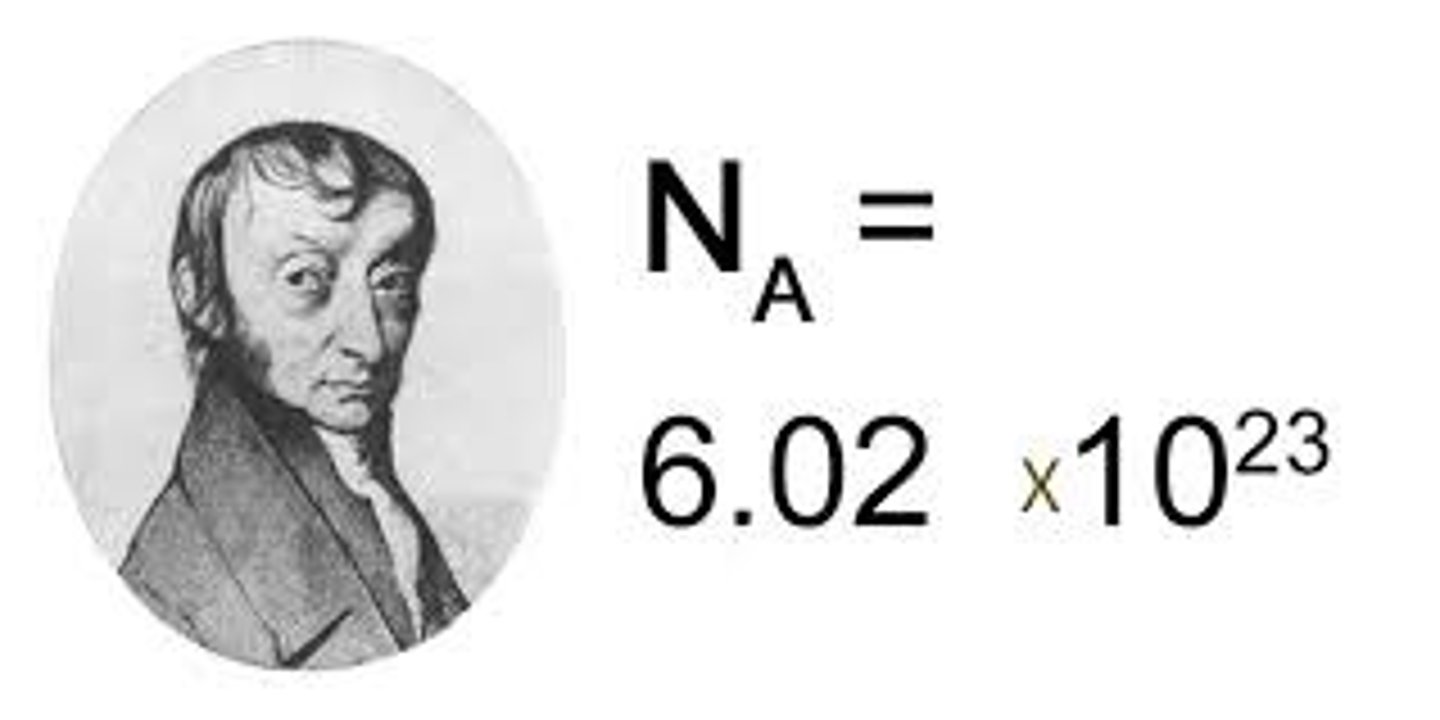
what is the atomic molar mass of an element
the atomic molar mass of an element is the mass in grams of one mole of atoms of that element
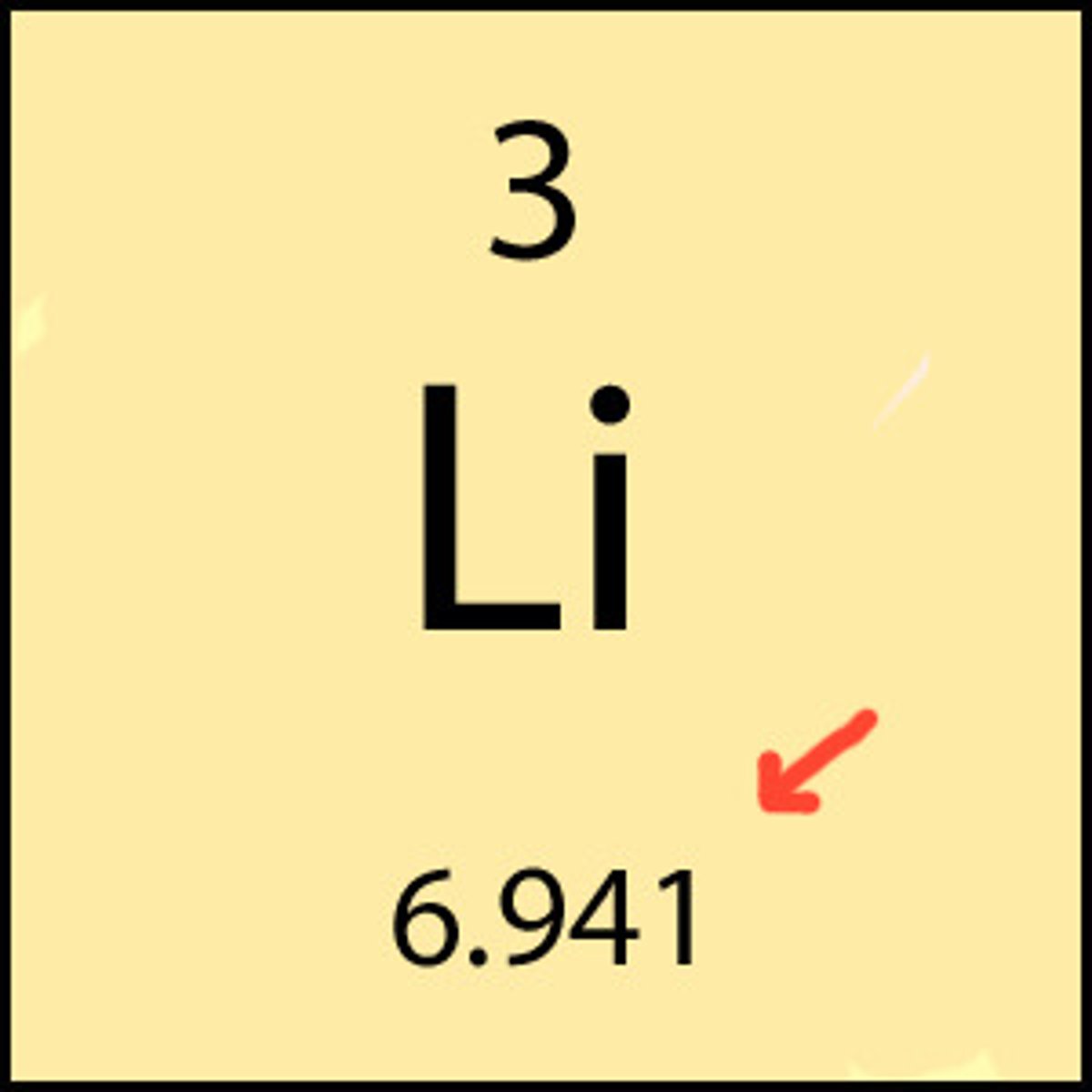
how to calculate the molar mass of a compound
add together the atomic molar masses of all the atoms in the compound (molar mass is written to 2 decimal places)
what is the factor-label method
use molar mass as a conversion factor between mass (g) and chemical amount (mol)
how to figure out the number of moles in a sample of an element
mass of the sample in grams x mol/g = moles of element. Ex w Ge: 15.4g x (1 mol/72.63g/mol) = 0.212 mol
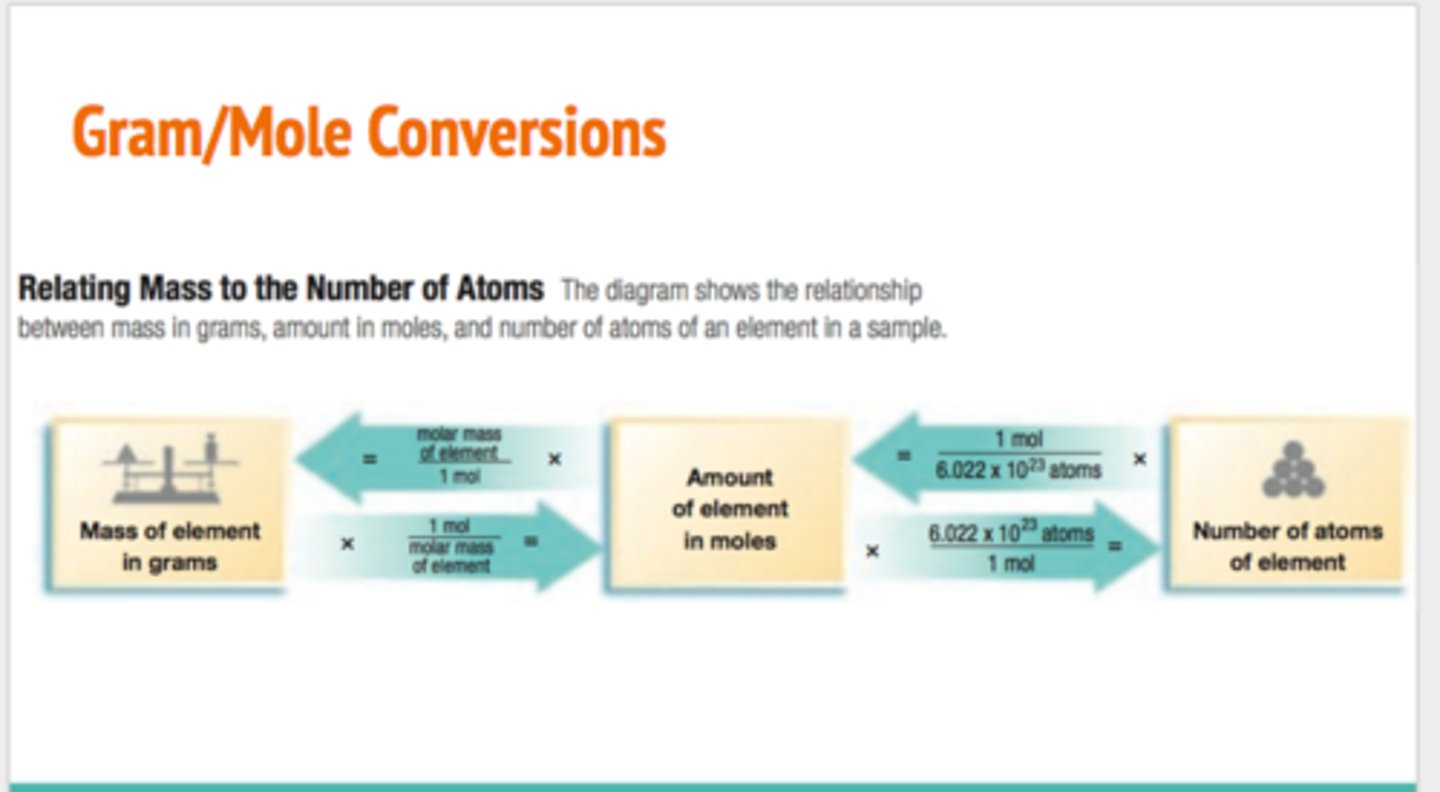
how to figure out the number of atoms in a sample of an element
# of moles x avogadros # = atoms. Ex w Ge. 0.212 mol x (6.02x10^23/1 mol) = 1.28x10^23 atoms
how to convert from # of atoms to grams?
(# of atoms in sample/1) x (1 mol/ 6.02x10^23 (avs number) x (molar mass of element in sample/ 1 mol)