Lecture 18 Metabotropic Receptors
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
Comparing the structure of ionotropic and metabotropic receptors
Ionotropic:
Ach specifically- has 5 different subunits with 4 diffferent domains- all come together to make recpetor
metabotropic receptor: no subunits, one protein
7 transmembrane segments- all of them
amino terminus extracellular, carbon - inner membrane
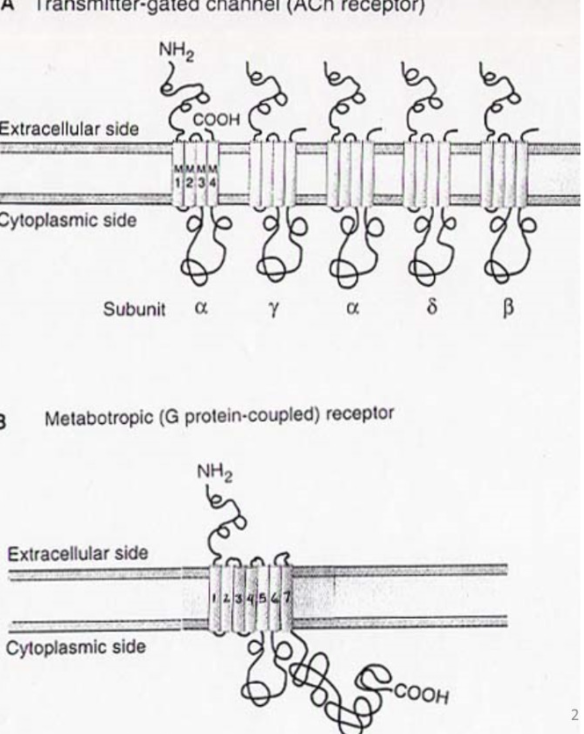
Info about metabotropic receptors - what segments are best conserved and why
intracellular part has g-protein binding domain
6-7th transmembrane segment
This means that this part of the channel has a good amount of conservation because all g proteins bind here in diff metabotropic receptors
RECEPTOR IS NOT AN ION CHANNEL!
does not pass current and no pore for things to go through
it transduces extracellular signal to intracellular
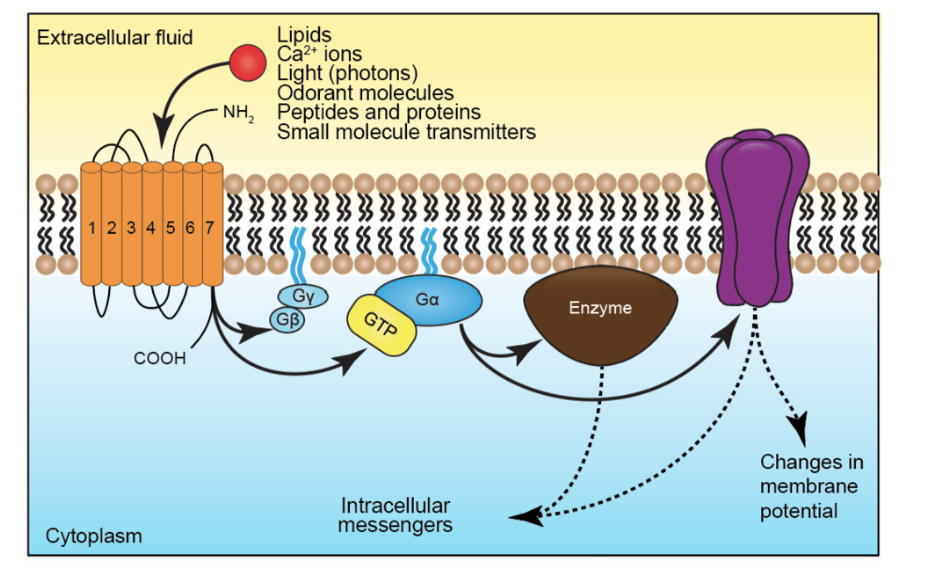
Structure of metabotropic channel- g proteins - what can it do to ionotropic channels
g-proteins are heterotrimeric- has alpha, beta, gamma components
GTP binding proteins- bind to GTP and
LIGANDS: many neurotransmitters for ionotropic also bind to metabotropic
activation of metabotropic receptor can modulate the activity of ion channels:
changes in membrane potential
change amount of transmitter release
change shape of AP
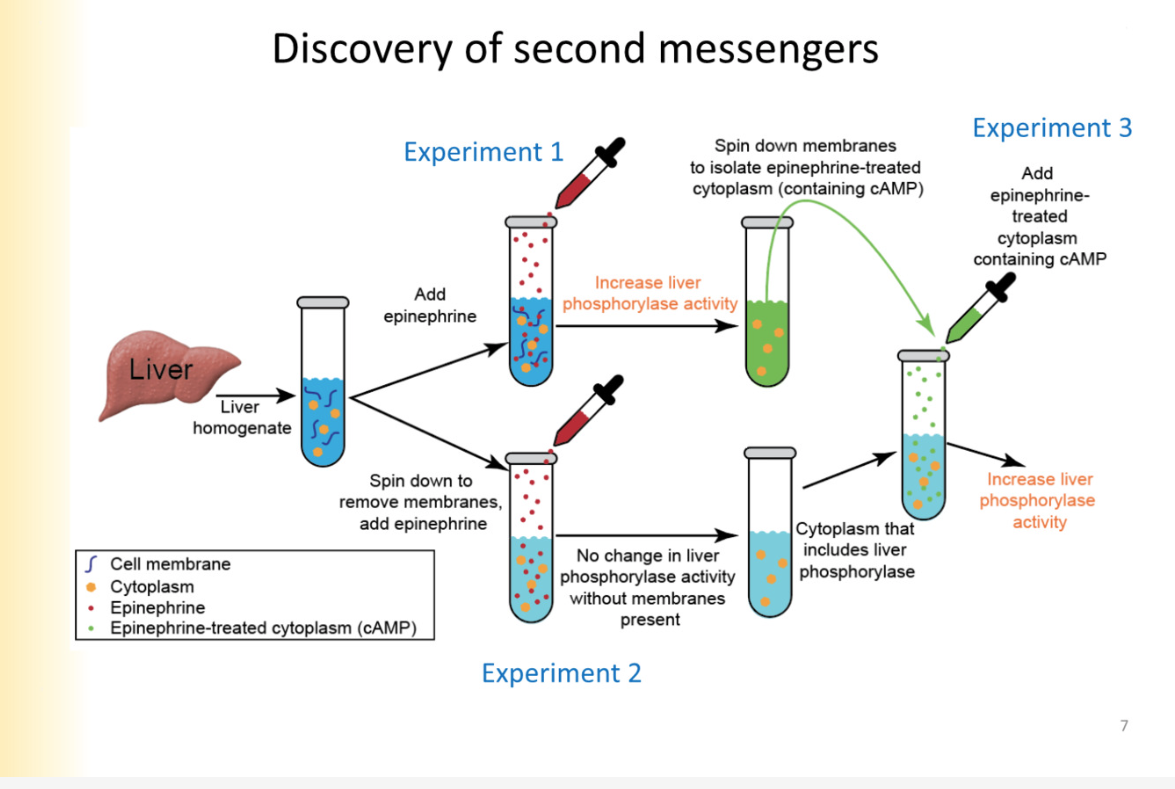
Discovery of second messengers experiment pt 1,2,3
transduce extracellular signal and connect it to intracellular response
Experiment:
observed activity of liver phosphorylase (enzyme)
increase and decrease activity based on hormones and neurotransmitters
measure activity in test tube- convenient
Make liver homogenate ( mixed up tissue)— no in tact cells there anymore
add epinephrine
increases liver phosphorylase activity
Experiment 2
spin in centrifuge
memrbane pieces are removed from solution bc they are heavy
have all components of cytoplasm
add epinephrine
no change in liver phosphorylase activity
Experiment 3:
take some epinephrine-treated cytoplasm
increase in liver phosphorylase activity
MEANS: epinephrine did something to the membrane that resulted in a cytoplasmic messenger that can modulate LP function
What is actually happening in liver cells when epinephrine is added
G- proteins are activated
activate adenylyl cyclase
cAMP
PKA- protein kinase A
liver phosphorylase
*epinephrine does not enter cell
not opening an ion channel
results in activation of intracellular cytoplasmic signalling molecule
G-proteins involved in activation of metabotropic receptors
When receptor not bound by ligand
alpha, beta, gamma subunits are all bound together
G alpha associated with GDP
before GDP turns into GTP, present in low concentrations in the cell (at rest)
g protein high affinity for GDP (alpha subunit)
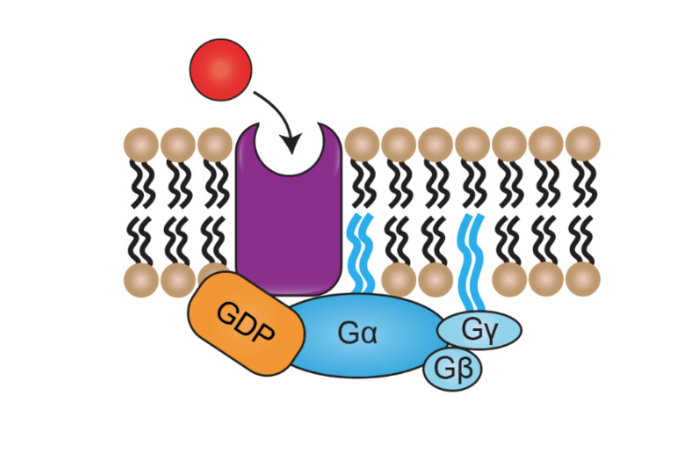
Activation of g-proteins step by step
ligand binds, causes conformational change in receptor protein
changes g-alpha affinity for GDP- now high affinity for GTP
EXHCHANGE: as soon as affinity for GDP swtiches, g-alpha binds to GTP
now g-protein is activated— can go do its individual stuff- can separate into its individual components
G-alpha GTP can be signalling molecule
G-beta gamma dimer can also be signalling molecule (do not come apart)
one or all of them can be doing things- gives variety to signalling pathways
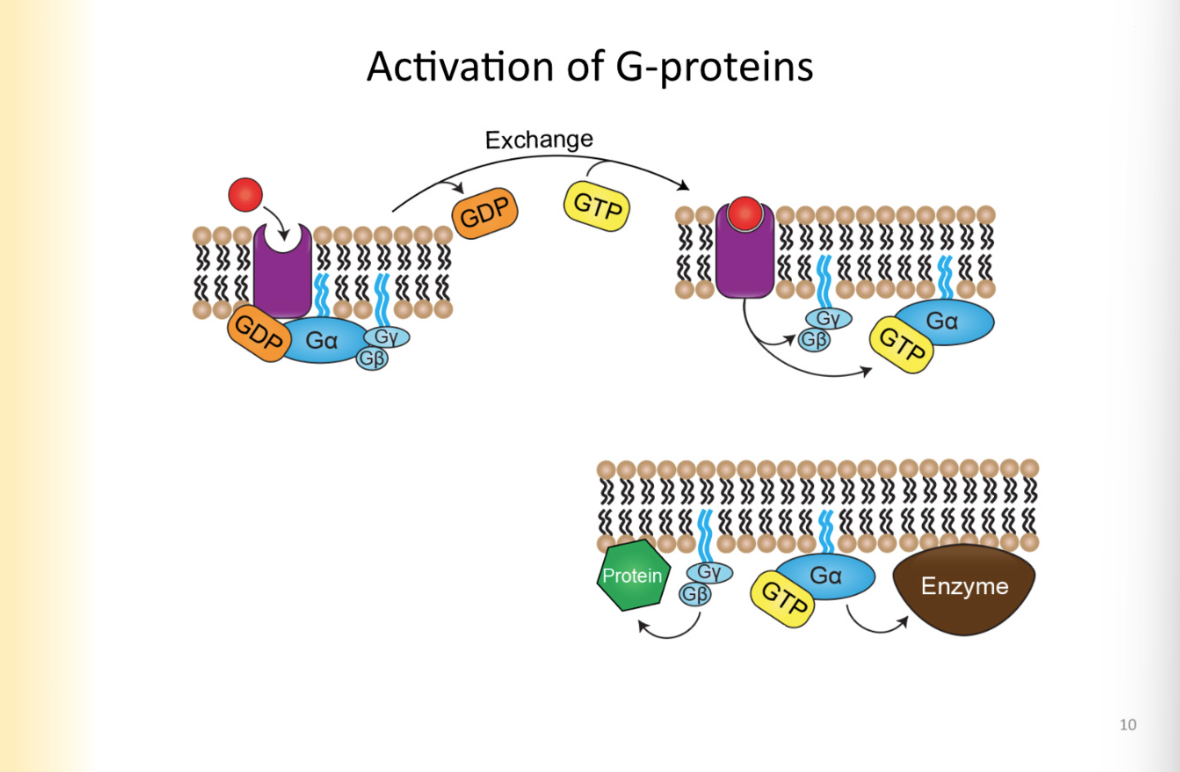
Which g-proteins remain associated with the membrane-what is the significance of this
look at slide
after activation as well
they are NOT fixed, they can move within the membrane
stay associated with membrane in general region by receptor
non-soluble cytoplasmic proteins
There are so many
Sig:
so many signalling pathways in the cell
each activated by g-proteins
if you apply NE to cell, and activates NE receptors, g protein should NOT turn on all the pathways
that is why it is important that it has a general vicinity
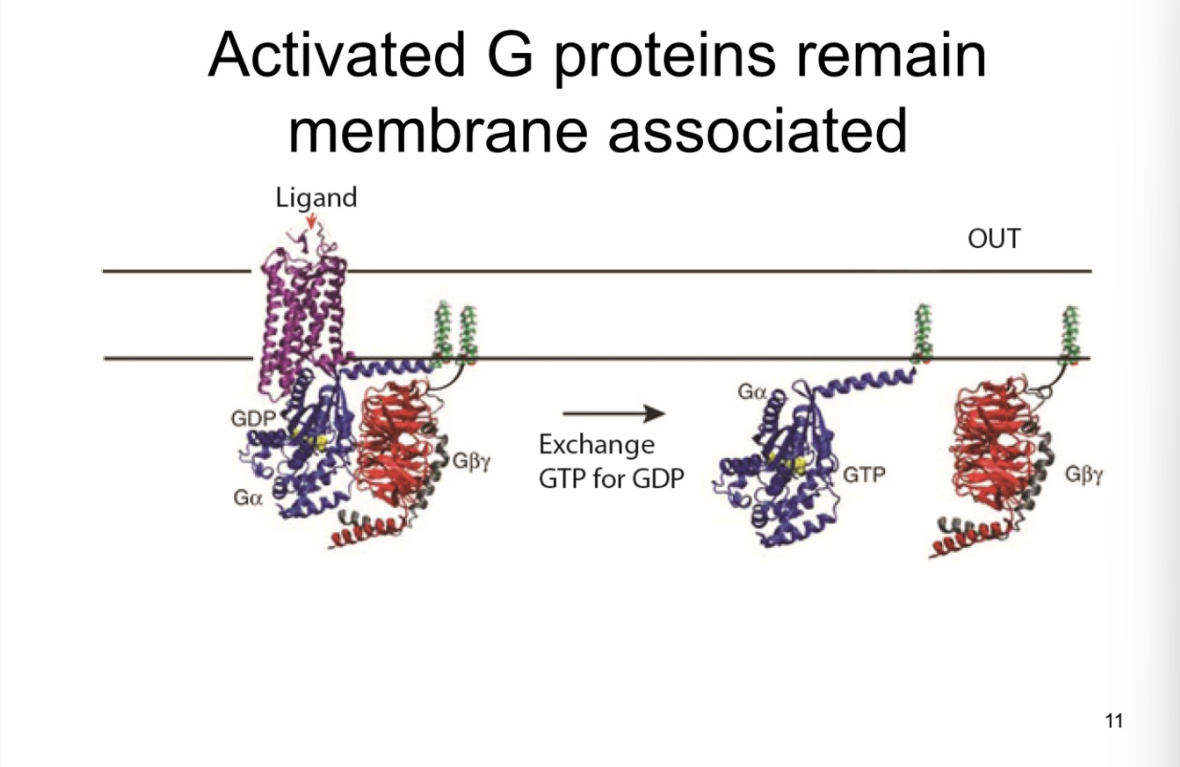
Inactivation of G-protein and time vs ionotropic receptor
** Not the opposite of activation
G-alpha subunit has INTRINSIC gtp-ase activity
means GTP-ase hydrolyzing GTP
hydrolysis changes GTP to GDP
G-alpha has high affinity for GDP
reassociates with membrane receptor
back to resting state
metabotropic effects last longer and takes longer
secondary reaction to the activation of g proteins lasts longer than the receptor inactivated
for iontropic receptors, after channel done with conducting ions, the effect is not seen
seconds vs a few miliseconds to open channels
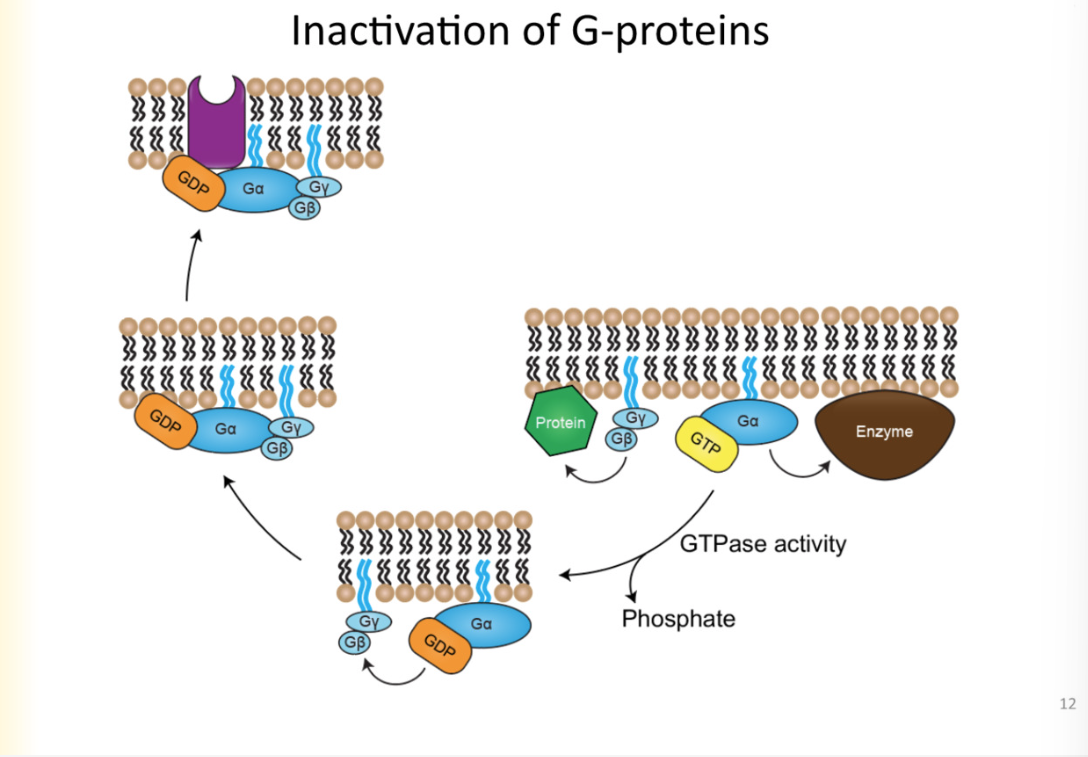
Modulation of metabotropic termination
GTP to GDP hydrolysis is normally slow
sped up sometimes through g-alpha molecule binding to effector
Regulation of G-protein signalling (RGS)- they are not effectors, they regulate GTPase activity of galpha
they know how long g-protein signalling lasts
Experiments to demonstrate role of GTPS
WHOLE CELL EXPERIMENT
cell that you are studying will be replaced by contents of the patch electrode
do not put GTP in patch clamp to eliminate it
voltage clamp experiment - depolarize and turn on ca2+ current
apply neurotransmitter
ca2+ current decreases in amplitude
is it GTP mediated?
methods:
remove GTP from intracellular solution
use GTP-gamma-S (non-hydrolyasable form of GTP)
USe GDP-beta S (high affinity for alpha subunit)
Without GTP, g-proteins cannot do anything
if you no longer get signal without GTP, g proteins are required in cell
With GTP-gamma S
once GTP is activated, it is on forever
cannot turn the signal off
GDP beta S
will replace endogenous GDP for binding to Ga
will not exchange for GTP
oppsite affect of GTP gamma S
what is crosstalk
crosstalk- one NT activating everything
what is the source of variability for G-proteins and how to find which different g-proteins are coupled to which different receptors
there are multiple types of alpha, beta and gamma subunits in g proteins
like a handful
one type of receptor coupled to a specific type of alpha, beta, gamma
HOW TO IDENTIFY DIFF GPROTEINS:
insert antisense strand (opposite of mRNA into the cell)
antisense to mRNA for protein
around 30 pairs long
binds to mRNA so it is no longer single-stranded
double stranded mRNA cannot be translated
cell does not make protein anymore
wait a day or two to have previously made protein expire/degrade
activate g-protein coupled receptor- see if still works
IF Gene you chose to do mRNA from was caused activation, signalling pathway wont work now**
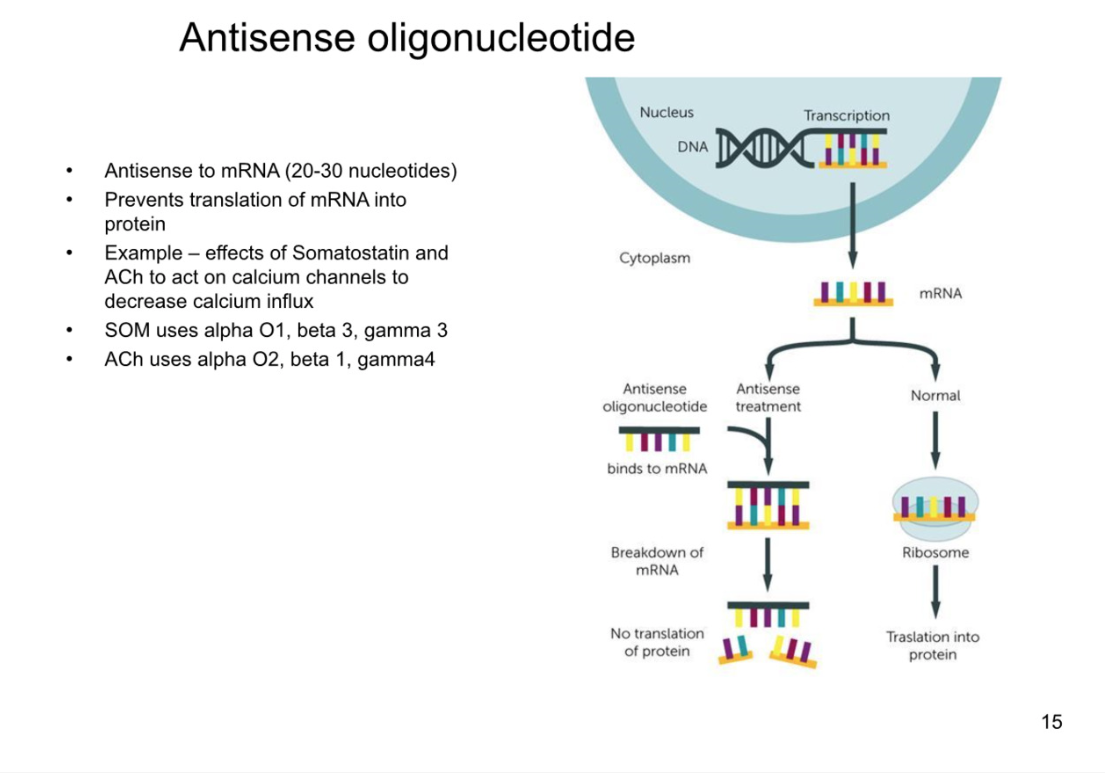
Spatial segregation of G-protein subunits
macromolecular complex- all within same vicinity
because the g-proteins cannot move large distances from where they are intially hinged, they cannot activate any other complexes they are not directly coupled with
can avoid cross talk within cell
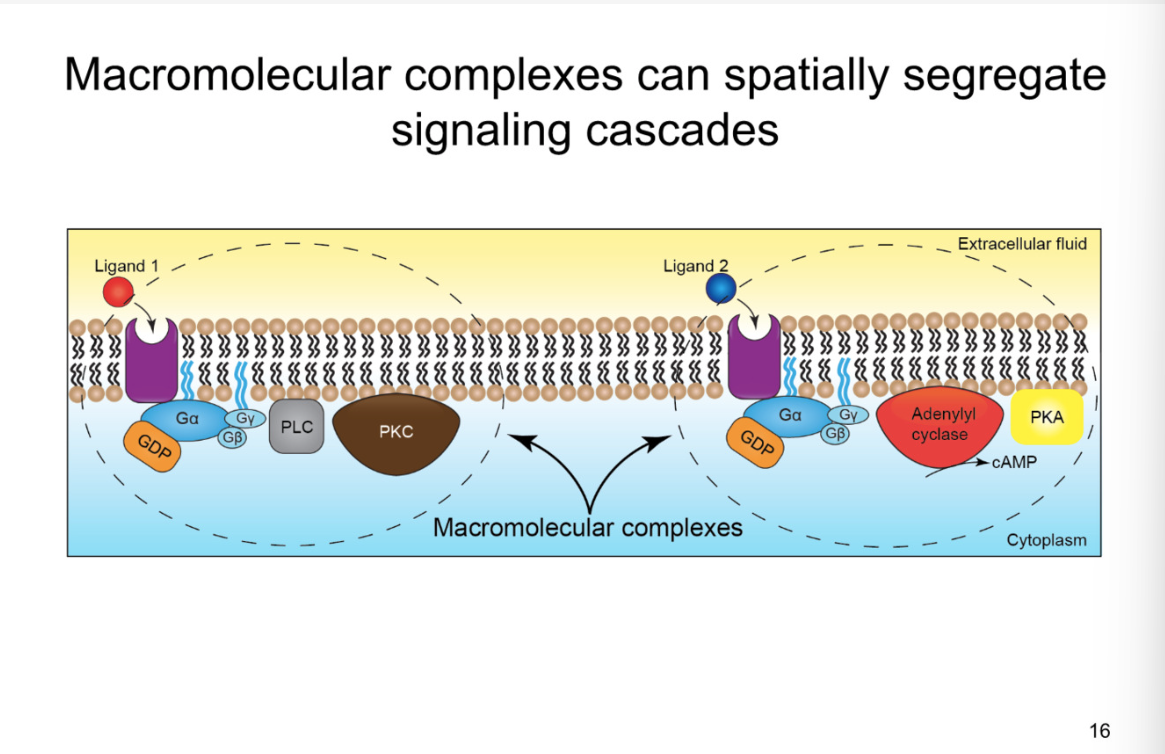
Metabotropic receptors can cause changes in membrane potential
picture- autonomic sympathetic synapse
synapse between preganglionic and post
synapses also from spinal cord that synpases on diff type of cell (C cell)
From sympathetic chain ganglia:
synapse releases Ach
effect: bind to nicotinic ach recpetors (ionotropic)
results in EPSP
B cell:
metabotropic receptors located at perisynaptic and extrasynaptic sites
high affinity for neurotransmitters- do not need to be in the cleft
both have negative effect on potassium channels- depolarizing the cell
Muscarinic ach receptors
LHRH receptor
K+ channels- CLOSE
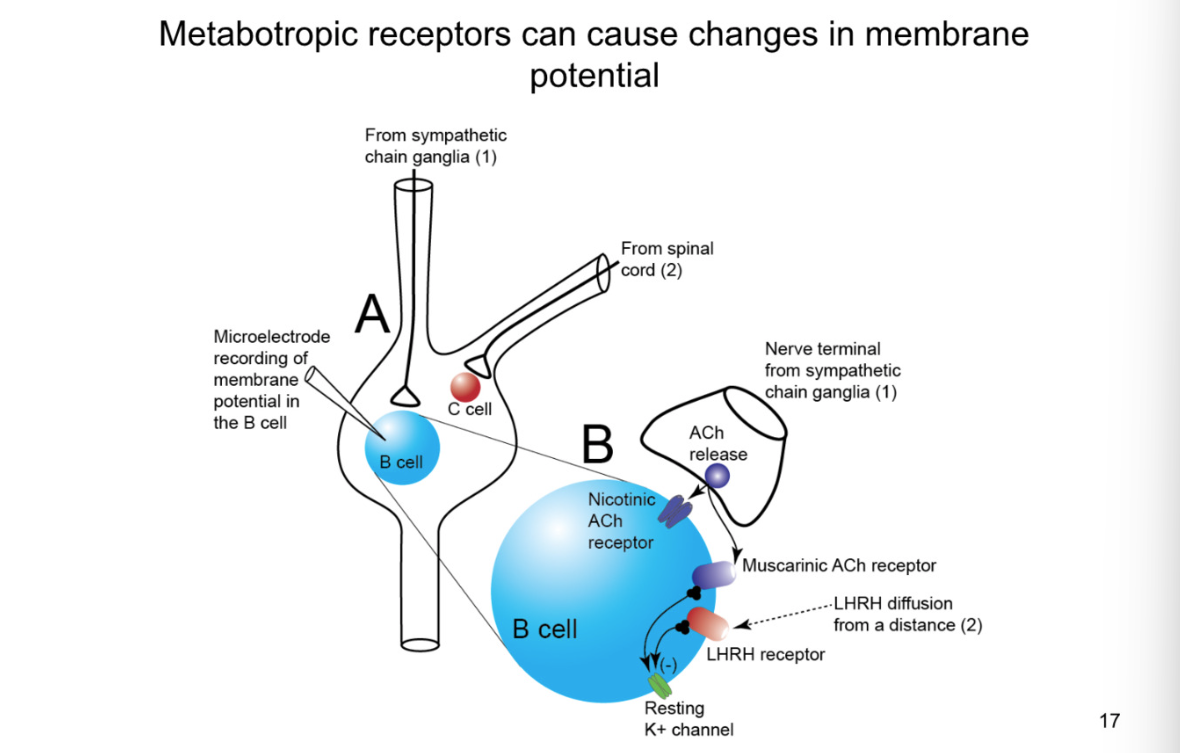
How do you activate the metabotropic receptors on the b b cell and what does the EPSP look like
look at chart to see what happens with stimulation to other cells
when stimulate B cell with ionotropic signalling at HGH FREQUENCY get enough acetylcholine release that it can diffuse out of the synpase and activate metabotropic receptors
close k+ channel, slow EPSP
shows that ionotropic fast and other is low
causes slow ionotropic action potentials as well
Stimulate 2:
At even higher stimulation: release NT and dense core neurotransmitters
late, slow EPSP- more action potentials and more diffusion for LHRH to reach its receptors
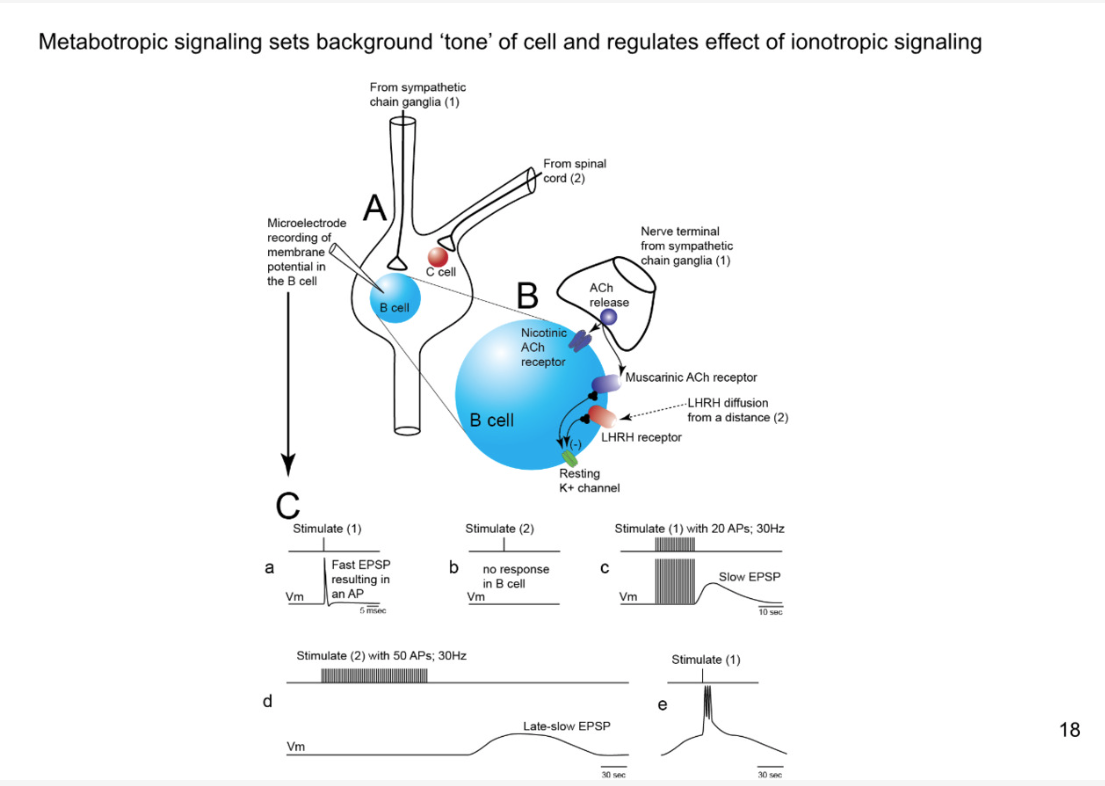
What is value of late, slow EPSP
stimulate 1 during late and slow EPSP- you will get a train of action potentials instead of just getting one
it increases the strength of synapse
there are many situations where you need significant activation of fight or flight