Biology - Chapter 2 Biological Molecules
1/40
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
What is biochemistry?
Why is it important?
How is it controlled?
State an important feature of biochemistry.
Biochemistry is the chemical reactions of biomolecules.
The sum of all biochemical reactions that take place in the body is called ‘metabolism’.
Having a limited variety of biomolecules makes metabolism easier to control.
There is a close link between the functions of molecules and their structures.
What are the 4 most common elements found in biomolecules in order of abundance?
Which is the most important? Why?
What do these elements make up?
Hydrogen > Carbon > Oxygen > Nitrogen
Carbon is the most important:
Many atoms join together to make long chains/ ring structures.
Covalently bonded C atoms make up the basic skeleton of biomolecules
Other atoms with different functions join to the skeleton.
They make up simple biomolecules which are used as the basic building blocks to make up larger molecules.
State the main building blocks of life and the complex molecules they form.
monosaccharides → polysaccharides (and used to make nucleotides).
Organic (nitrogenous) bases → nucleotides → nucleic acids
amino acids → proteins
fatty acids + glycerol → lipids
Define the following:
Macromolecule
Polymer
Monomer
Give examples of covalent bonds in biomolecules.
A large molecule (polysaccharides/proteins/nucleic acids)
A giant molecule made up of many similar repeating subunits linked up to make a long chain. These subunits are smaller and simpler in structure and are called monomers. polysaccharides/proteins/nucleic acids are examples of polymers.
A relatively simple molecule used as a building block for the synthesis of polymers. Many monomers are joined together by covalent bonds in a condensation reaction to make polymers. Examples include: monosaccharides, amino acids and nucleotides.
Covalent bonds (sharing of e-): Glycosidic bonds, ester bonds and peptide bonds.
Why is making polymers a relatively simple process?
Define condensation reactions and hydrolysis reactions.
Because it’s the same reaction repeated many times.
Condensation reactions:
A reaction involving the joining together of 2 molecules by the removal of a water molecule.
Hydrolysis reactions:
A chemical reaction in which a chemical bond is broken down by the addition of a water molecule. This is typically used when a complex molecule is broken down to a simple one.
Define monosaccharide.
A monomer consisting of a single sugar unit. It dissolves in water to form a sweet-tasting solution. It has the general formula (CH2O)n
State the different types of monosaccharides.
Hexoses (C6H12O6)
Glucose, fructose, galactose
Pentoses
Ribose, deoxyribose
Triose
What are the 2 isomers of glucose and their structures?
Alpha (hydroxyl group below plane) and Beta glucose (hydroxyl group above plane)
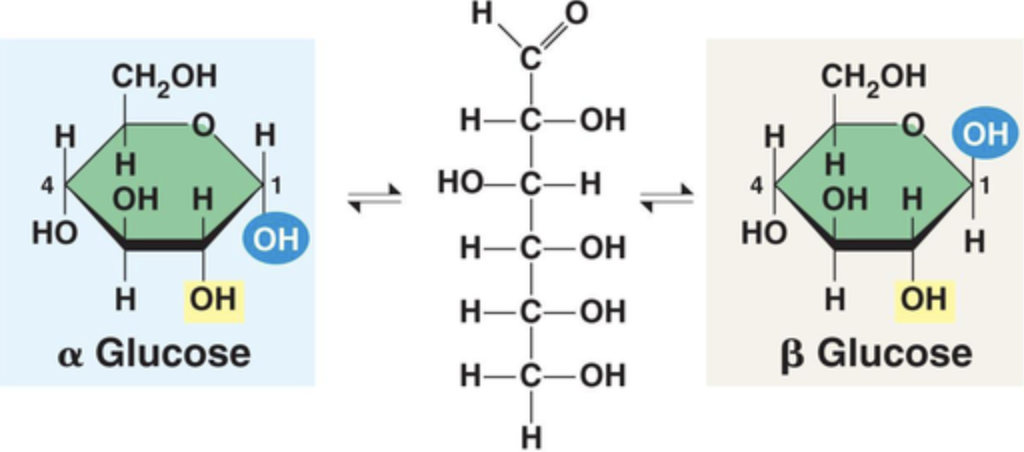
What are the major functions of monosaccharides?
Source of energy in respiration (most important source for energy metabolism = glucose).
Due to the large no. of C-H bonds, which when broken releases a lot of energy.
The energy is transferred to make ATP from ADP and a phosphate group during respiration.
Used as building blocks for larger molecules:
Polysaccharides (cellulose, starch, glycogen)
DNA and RNA from deoxyribose and ribose.
Which monosaccharides join us to make which disaccharides?
What are these reactions examples of? Explain the reaction.
How is it made sure that only a limited variety of the same substance is made.
Glucose + Glucose → Maltose
Glucose + Fructose → Sucrose
Glucose + Galactose → Lactose
Condensation reactions - reverse process is hydrolysis.
2 -OH grps line up alongside each other
One -OH grp combines with the H from the other grp to form water
This allows an ‘O’ bridge to form between the 2 molecules, holding them together.
This forms a disaccharide and a glycosidic bond.
Any 2 -OH grps can line up. But, the shape of the enzyme controlling the reaction determines which -OH groups can come alongside each other.
Define polysaccharides and state which are the polysaccharides of glucose.
A polymer whose subunits are monosaccharides are linked together by glycosidic bonds, formed through a condensation reaction.
Starch, glycogen and cellulose. None of which are sugars.
Why is glucose stored as starch/glycogen?
glucose main source of energy for living cells
must be stored in appropriate form
glucose can be dissolved, so if it is accumulated in cells, it can seriously affect the osmotic balance (inside of cells become very conc.) and it is also reactive molecule and can interfere with normal cell chemistry.
As a polysaccharide, it is compact, inert, insoluble and convenient.
Plants store as starch and in animals it is stored as glycogen
When needed, glucose is quickly made available again by enzyme-controlled hydrolysis reactions.
Describe the structures of starch.
Starch is a mixture of amylose and amylopectin.
amylose is made from condensation reactions joining many a-glucose in a long unbranching chain of 1,4 linked glycosidic glucose molecules built up.
The chains are curved and makes it coil up into a helical structure that is compact.
amylopectin is also is made from condensation reactions joining many a-glucose in short unbranching chains of 1,4 linked glycosidic glucose molecules built up.
But they also contain 1,6 glycosidic links that start branches out of the side of the chain.
Mixtures of amylose and amylopectin build up to make relatively large starch grains that are stored in chloroplasts/storage organs (tubers/seeds of cereals)
Describe the structure of glycogen.
It has a very similar structure to amylopectin:
It has 1,4 a-glucose links using glycosidic bonds, with 1,6 a-glucose glycosidic links creating branch points.
Glycogen is more branched than amylopectin.
Glycogen molecules clump up to form large granules seen in liver cells (energy reserve).
Why is cellulose the most abundant organic molecule?
What role does it play?
Due to its presence in plant cell walls and its slow breakdown in nature
A structural role as it is mechanically strong.
Describe the structure of cellulose.
made of 1,4 beta-glucose glycosidic linkages to form an unbranching chain.
The -OH grp in beta-glucose is above the plane on C-1 and below the plane in C-4
The -OH grps of C-1 and 4 must line up alongside one another for the condensation reaction to take place.
This means successive glucose molecules must be rotated 180 degrees relative to the other.
Explain how the structure of cellulose molecules relates to its function.
The arrangement of beta-glucose molecules results in a strong overall molecule:
The H atoms in the -OH grps are weakly attracted to the O atoms in the same molecule and O atoms in OH grps of neighbouring molecules.
H-bonds are individually weak, but many of them collectively provides enormous strength.
How are cellulose molecules arranged in the cell wall of plant cells? and how does it relate to its function.
60-70 cellulose molecules becomes tightly cross-linked by many hydrogen bonds to make bundles called microfibrils.
Microfibrils are held together by H-bonding to form bundles called fibres.
Cell walls have many layers of fibres that run in diff. directions to increase strength.
Other substances help cross-link fibres/form a glue-like matrix around fibres which also increases strength (pectin).
Has high tensile strength (withstand high pressures developed inside due to osmosis) - without wall cell would burst in dilute solution.
Pressures provide support for plant (makes tissues rigid + cell expansion during growth)
Arrangement of fibres allows the determination of cell shape.
Freely permeable.
How are hydrogen bonds formed?
In covalent bonds, atoms share a pair of electrons.
In some molecules, electrons are not shared equally, one of the atoms may pull the pair of electrons towards itself (getting a partial negative charge). H atom usually gets partial +ve charge.
Unequal charge distribution = dipole.
The +vely charged H atom will be attracted to a -vely charged atom of a neighbouring molecule.
Molecules having groups with dipoles = polar (attracted to water molecules - hydrophilic + soluble)
Molecules with no dipoles = non-polar (not attracted to water molecules - hydrophobic + insoluble).
What are lipids?
Organic molecules that are insoluble in water
They are formed from a reaction between fatty acids and alcohols.
They are classified into fats (solid) and oils (liquid).
Describe the constituents of lipids.
Fatty acids
Carboxylic acids with the functional group -COOH (carboxyl grp)
The carboxyl grp makes the up the head of the fatty acid tail.
Attached to the -COOH grp is a long hydrocarbon chain/tail.
If the tail has double bonds it is unsaturated (they don’t contain the maximum possible amount of H atoms):
One double bond = monounsaturated
More than one double bond = polyunsaturated
If the tail consists only of single bonds = saturated -usually fats (animals)
Unsaturated is more healthier (as it is more reactive and melts easily) - usually oils (plants)
Alcohols
Contains functional group -OH
Most common alcohol is glycerol which contains 3 -OH groups.
HOW ARE LIPIDS PRODUCED?
Fatty acids and the alcohol reacts together to form a lipid (also called an ester).
The chemical link between alcohol and acid is the ester linkage (-COO-)
This is also a condensation reaction - water formed as product.
Hydrolysis is the reverse reaction of breaking fat down into acid + alcohol by adding water.
Describe and explain triglycerides.
Most common lipid
Formed by the reaction between glycerol (3 -OH groups) and 3 fatty acids.
Each -OH grp of glycerol undergoes a condensation reaction with a fatty acid
Final molecule
1 glycerol
3 fatty acid tails (can vary in length)
3 ester bonds
Insoluble in water, soluble in organic solvents like ethanol (because hydrocarbon tails are non-polar)
Lipids are hydrophobic and are immiscible with water.
State the functions of triglycerides
Excellent energy stores
Has more C-H bonds than carbs
Stored under skin, around kidneys…
Acts as an insulator against loss of heat
Provides buoyancy
metabolic source of water (when oxidised in respiration, triglycerides produces CO2 and H2O).
Offers protection to organs.
Describe phospholipids and their main function.
One of the 3 fatty acids of a triglyceride is replaced by a soluble (hydrophilic) phosphate grp.
This phosphate grp is polar and can therefore dissolve in water (one end of phospholipid is soluble) - head of phospholipid is soluble.
The 2 remaining hydrocarbon tails are still hydrophobic.
This is useful in the formation of membranes around a cell.
2 rows of phospholipids where the hydrophilic heads are in the outer surface in the watery solution on either side of the membrane.
The hydrophobic tails create a layer impermeable to hydrophilic substances.
State 9 functions of proteins.
Enzymes are proteins
Essential components of cell membranes
Some hormones (insulin/glucagon) are proteins
Oxygen-carrying pigments; haemoglobin (Hb) and myoglobin.
antibodies
collagen adds strength to many animal tissues (bones, and blood vessel walls)
Hair, nails, surface of skin contains keratin
actin and myosin responsible for muscle contraction
storage products (casein and ovalbumin).
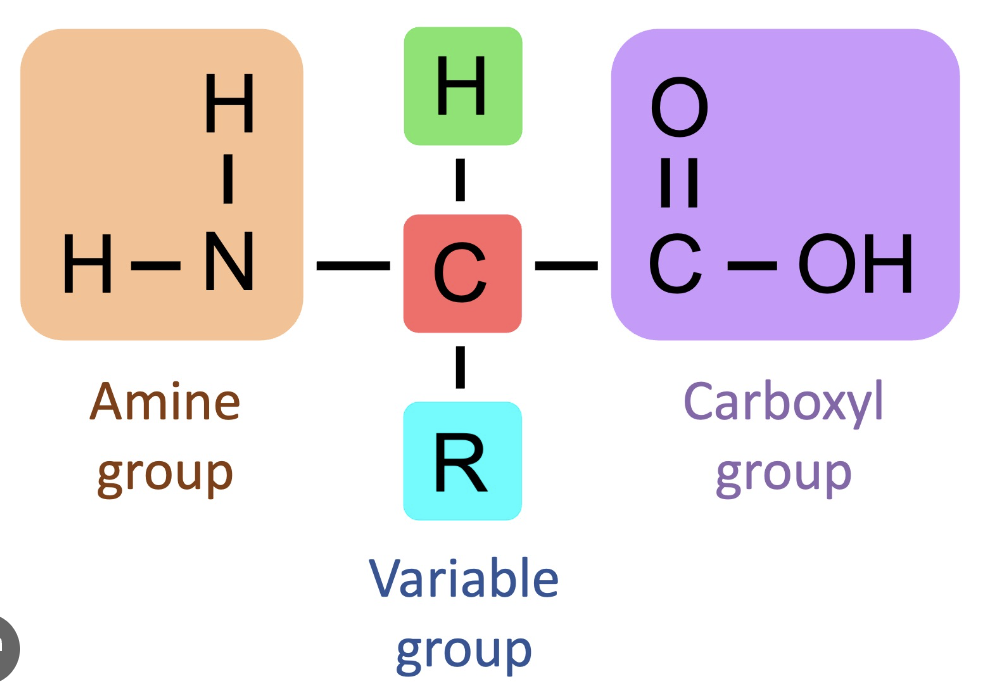
Describe the structure of amino acids.
The only way amino acids differ from one another is through the 4th grp - the R group.
There are 20 different a.as
How do peptide bonds form?
2 amino acids join together:
1 loses -OH grp from -COOH grp
The other loses H from -NH2 grp.
The C-atom of 1st aa, bonds with N-atom of 2nd aa.
This is a peptide bond.
The -OH and H removed forms water molecule - condensation reaction.
2 aa joined together by peptide bond = dipeptide
Many aa joined together by peptide bonds = polypeptide (protein)
Describe the primary structure of proteins.
It is the sequence of amino acids in a polypeptide chain.
The sequence an type of amino acids used makes this up
Since there can be thousands of amino acids in a single polypeptide chain - even changing just one amino acid can alter the properties completely.
Define the secondary structure of proteins.
The structure of proteins resulting from the regular coiling/folding of a chain of amino acids (alpha helix/beta pleated sheet).
Describe the 2 structures of secondary structure of proteins.
amino acids in a polypeptide chain can have an effect on one another even if they are not next to one another in the primary sequence.
The primary structure can bend back on itself.
Alpha helix : a helical structure formed by a polypeptide chain (PP chain) held in place by H-bonds.
When PP chain coils into a corkscrew shape
H-bonding between O from CO and H from NH in an amino acid 4 places ahead of it.
Beta pleated sheet: A loose, sheet-like structure formed by the hydrogen bonding between parallel polypeptide chains.
*H-bonding is strong enough to hold these structures in place, but is easily broken by high temperatures and pH changes.
Define the tertiary structure of a protein.
The compact structure of a protein resulting from the three dimensional coiling of a chain of amino acids.
Describe the tertiary structure of proteins.
When the secondary structure itself is coiled/folded.
The shape of the molecules are very precise and are held in shape by bonds between amino acids in different parts of the chain.
What are the bonds that are used to keep proteins in their precise shapes?
Hydrogen bonding (between strong polar groups)
Weak in isolation, but strong when many together.
Disulfide bonds (between 2 cysteine molecules - contains S atoms)
When S atoms of neighbouring cysteines join together with covalent bond (strong)
removal of H = oxidation
gaining of H = reduction
disulfide bond broken by reducing agents
Ionic bonds (between ionised amino and carboxyl groups) broken by pH changes.
Weak hydrophobic interaction
Between non-polar R groups (hydrophobic, avoids water)
These groups stay together to expel the water (they are repelled by water)
Shape of proteins are affected by hydrophobic interactions.
R groups oriented towards centre of protein, facing away from outside watery environment + hydrophilic R groups surround them and point outwards in contact with water.
Define quaternary structure of proteins.
The three-dimensional arrangement of 2 or more polypeptide chains (or 1 polypeptide chain and a prosthetic grp) in a protein molecule.
Describe the 1st type of quaternary proteins.
Globular proteins: a protein whose molecules are folded into a relatively spherical shape. They often have physiological roles, are soluble and are metabolically active.
like myo/haemoglobin
They curl up into a ball shape so that the non-polar R-grps points toward the centre of the molecule (away from water)
Water molecules excluded from centre of molecule
Polar R grps point towards the outside of the molecule (globular proteins are soluble as water molecules cluster around the outward hydrophilic R grps).
They have functions in metabolic reactions, where their precise shape is key to their functioning.
Describe the 2nd type of quaternary proteins.
Fibrous protein: A protein whose molecules have relatively long and thin structures, where they are insoluble and metabolically inactive and have more structural roles.
Describe the structure of Hb.
A globular protein
It is an O2 carrying pigment
Made of 4 polypeptide chains (each chain is a protein called a globin)
2 types of globin used in Hb: alpha and beta.
2 chains (alpha) made of alpha globin
2 chains (beta) made of beta globin
Hb nearly spherical and 4 PP chains are packed closely together
Hydrophobic R groups point inwards, hydrophilic R grps point outwards
Hydrophobic interactions important in holding 3D shape.
hydrophilic R grps important to maintain solubility
Each of the 4 PP chains of Hb has haem group (prosthetic grp - important + permanent, not made of aa)
Each haem grp contains an Fe atom - 1 O2 can bind with each Fe atom.
Haem responsible for colour.
Describe structure of collagen.
Fibrous protein
Found in skin, cartilage, bone, blood vessel walls.
Has 3 PP chains, each in shape of helix
3 helical PPs are wound around one another, to form triple helix
3 strands held together by H-bonds/covalent
Every 3rd aa is glycine (smallest)
found on inside of strands and small size allows 3 strands to lie close to each other to form tight coil.
Each molecule of collagen interacts with parallel running collagen strands
covalent bonds forms between R groups of amino acids lying next to each other.
Cross-links hold many collagen molecules together side-by-side
This forms ‘fibrils’
The ends of parallel molecules are staggered to prevent weak spot from running right across the collagen fibril.
Many fibrils lie next to each other to form fibres (strong bundles)
Collagen = flexible but high tensile strength.
Collagen fibres lined up differently depending on the forces they must withstand.
Why is water important?
major component of cells
Provides environment for organisms living in water
Is a solvent, and therefore provides a medium in which life can evolve.
Due to hydrogen bonding, a lot of energy would be required to convert water into a gas and so remains liquid at room temp.
Important properties of water and its uses.
Universal solvent
chemicals dissolve in water if polar (water collect around it and separates them)
in solution molecules and ions are free to move and react with other chemicals
ideal as transport medium
life processes take place in solution
if non-polar, they are pushed together by water as water molecules are attracted to one another (hydrophobic interactions in protein and membrane structures increases stability)
High specific heat capacity
To raise temp, liquid particles must gain energy and move more rapidly
H bonds making water molecules stick to each other makes it difficult to make molecules move freely
bonds must break to allow free movement - more energy needed to raise temp.
water resistant to changes in temp. - temp in organisms is relatively constant compared to changing environment around.
biochemical reactions happen at constant rates
stable habitats
high latent heat of vaporisation
heat needed to change l → g
consequence of high SHC
large amounts of energy needed to vaporise into gas (break H bonds)
E transferred to water, corresponds to loss of energy from surroundings - cools down.
evaporation used as cooling mechanism (sweating)
large amount of heat energy transferred for relatively little loss of water (reducing risk of dehydration)
cool leaves during transpiration
less likely organisms freeze.