CH.8 Understanding Solutions and Their Concentrations in Chemistry
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
What is a solution?
A system in which a solute is homogeneously mixed with a solvent.
What is the solute in a solution?
The least abundant component of the solution.
What is the solvent in a solution?
The most abundant component in the solution.
What are the common characteristics of solutions?
Solutions are transparent and homogeneous.
What is the general rule for predicting solubility?
Like dissolves like.
How do intermolecular forces affect solubility?
If solvent and solute have the same type of intermolecular forces, they mix more easily.
Which type of compounds are more soluble in polar solvents?
Polar compounds, including ionic compounds, tend to be more soluble in polar solvents.
Which solvent would dissolve NaCl better: H2O, CH3CH2OH, or C6H14?
H2O would dissolve NaCl the best due to its high polarity.
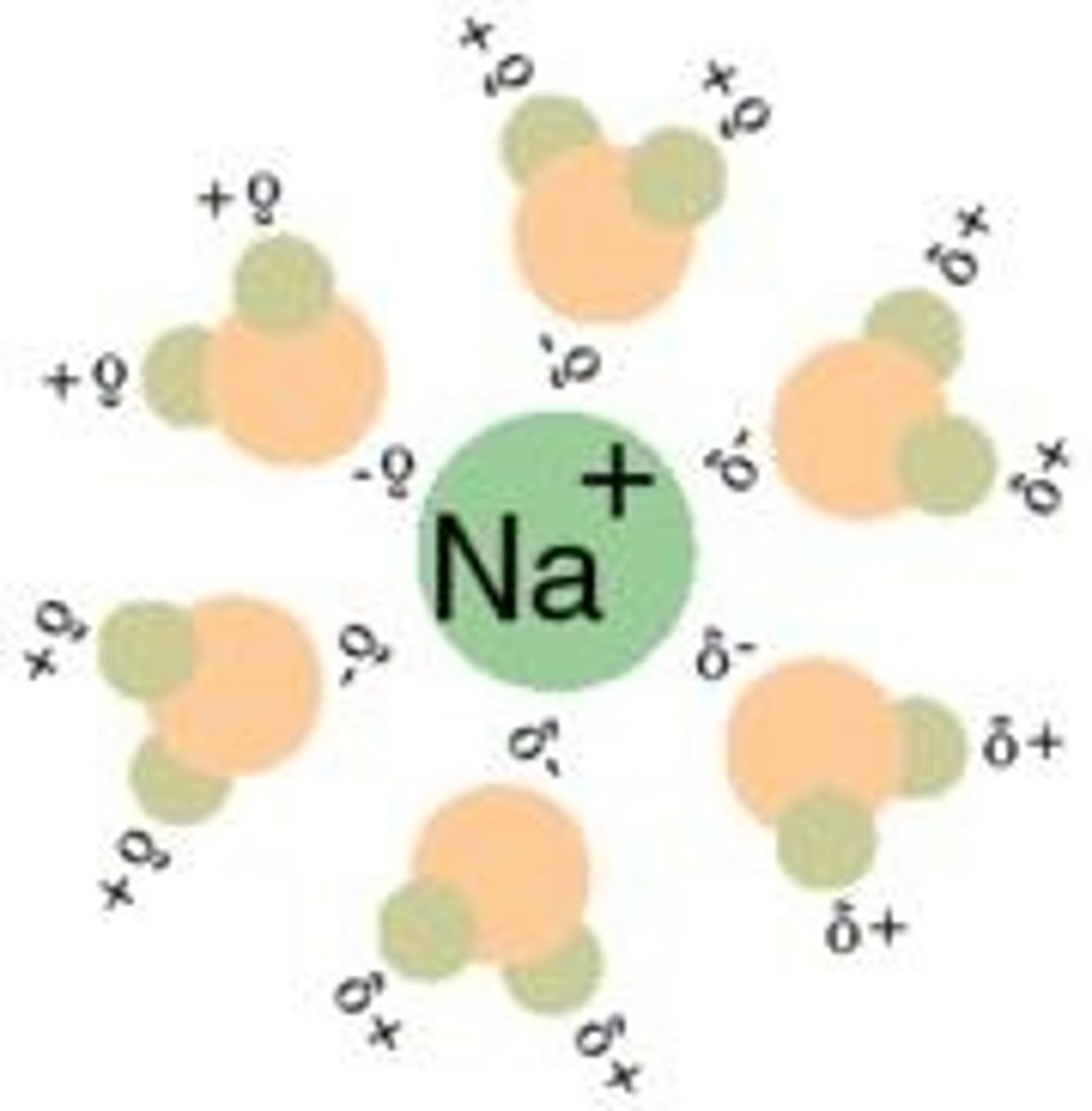
What is the solubility behavior of nonpolar compounds?
Nonpolar compounds tend to be more soluble in nonpolar solvents than in polar solvents.
What is mass percent?
Mass percent is the mass of solute divided by the total mass of solution multiplied by 100.
How do you calculate mass percent?
mass percent = (g solute / (g solute + g solvent)) x 100.
What is the mass percent of sodium hydroxide in a solution made by dissolving 8.00 g NaOH in 50.0 g H2O?
13.8% NaOH solution.
How do you find the mass of solute needed for a specific mass percent?
Use the equation: mass percent = (g solute / g solution) x 100.
What is mass-volume percent?
Mass-volume percent is the mass of solute (in grams) divided by the total volume of solution (in mL) multiplied by 100.
How do you calculate mass-volume percent?
mass/volume percent = (g solute / mL solution) x 100.
What is the volume percent?
Volume percent is the volume of solute divided by the total volume of solution multiplied by 100.
What is molarity?
Molarity is the number of moles of solute per liter of solution.
How do you calculate molarity from grams of solute?
Molarity = (number of moles of solute / liters of solution).
How do you convert grams of solute to moles?
Moles of solute = (g solute / molar mass g).
What is the molarity of a solution containing 1.4 mol of acetic acid in 250 mL of solution?
5.6 M.
How do you calculate the grams of KOH needed for a specific molarity?
Use the formula: grams KOH = (molarity x liters of solution x molar mass KOH).
What is the formula for dilution?
MstockVstock = MdilVdil.
How much of a 6.00 M HCl solution is needed to prepare 250.0 mL of 0.500 M HCl solution?
20.8 mL of stock solution is needed.
How do you calculate the molarity of a mixed solution?
Use the formula: (V1)(M1) = (V2)(M2) to find M2.
What is the molarity of a sodium hydroxide solution prepared by mixing 100 mL of 0.20 M NaOH with 150 mL of water?
0.080 M NaOH.
What type of compound is likely to dissolve in water?
Polar compounds.
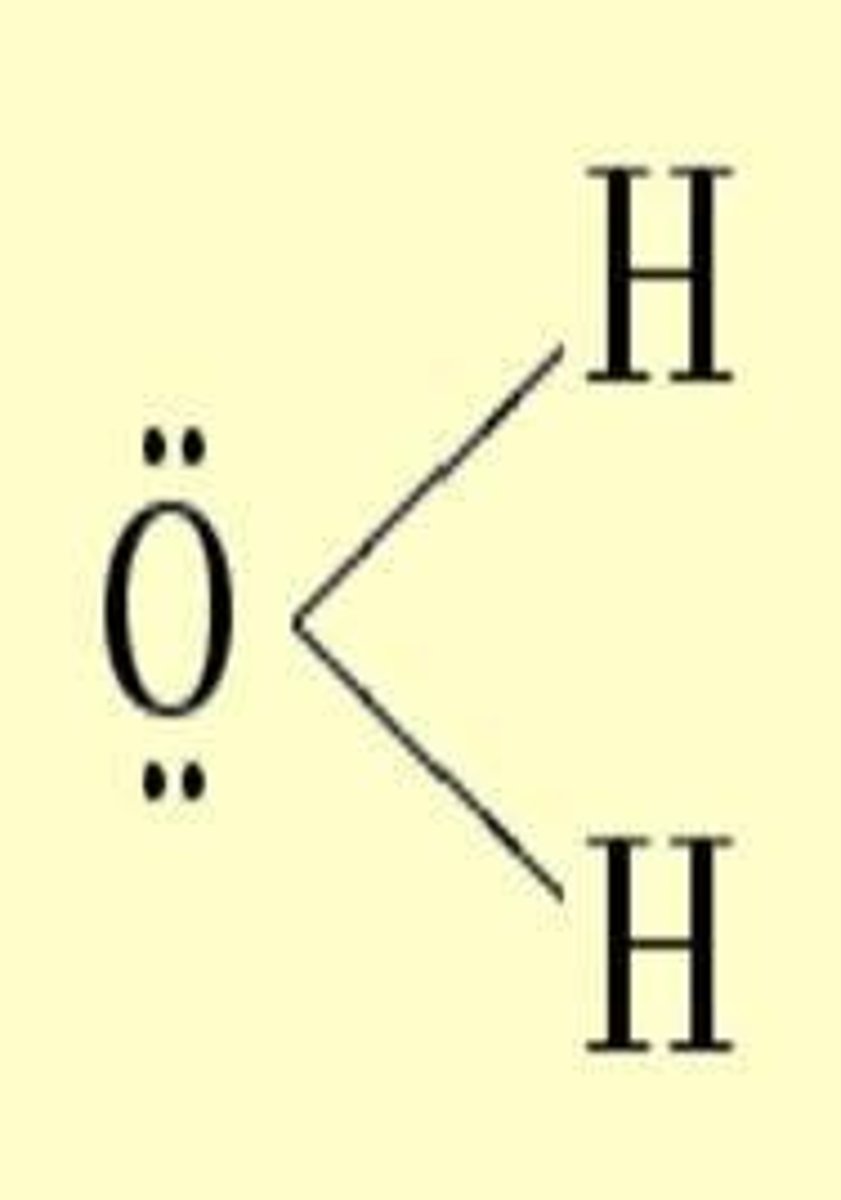
Which percentage concentration unit is most frequently used by chemists?
Mass percent.
How do you determine the amount of KCl in a solution with a concentration of 18% (m/v) in 36.0 mL?
Calculate using the formula: g KCl = (18/100) x 36.0 mL.
What is the concentration in mass-volume percent for 28 g CaF2 in 355 mL of solution?
Calculate using the formula: mass/volume percent = (28 g / 355 mL) x 100.
What mass of water is needed to prepare 200 grams of a 12.0% (m/m) KHCO3 solution?
Calculate using the formula: mass of water = total mass - mass of solute.
What is the molarity of a solution made by dissolving 3.18 moles of NaBr in enough water to give a final volume of 1030 mL?
Calculate using molarity = moles/volume in liters.
How do you calculate the volume of a 2.5 M solution needed to make 1.35 L of a 0.150 M solution?
Use the dilution formula: M1V1 = M2V2.