Crystal Structures
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
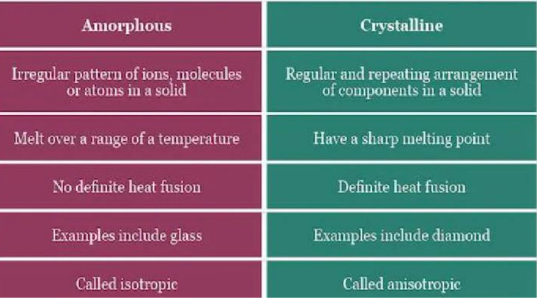
Crystalline Material
one in which the atoms are situated in a repeating or periodic array over large atomic distances.
tend to have sharp melting points.
Some of the common examples are:
diamonds
table salt
ice
sugar
most metals

Non-Crystalline / Amorphous Material
do not crystallize and the long-range atomic order is absent.
not organized in a definite lattice pattern such solids include glass, plastic, and gel.

Crystallography
the science of measuring the crystal structure of a crystal.
Crystals Structure
Some of the properties of crystalline solids depend on the crystal structure of the material, and the manner in which atoms, ions, or molecules are spatially arranged.
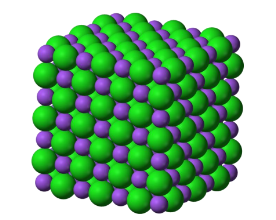
Atomic Hard-Sphere Model
spheres representing nearest-neighbor atoms touch one another.
Lattice
a three-dimensional array of points coinciding with atom positions (or sphere centers).
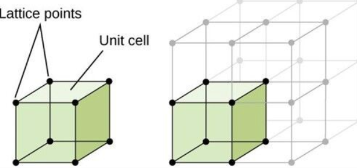
Unit Cell
small groups of atoms form a repetitive pattern
the basic structural unit or building block of the crystal structure and defines the crystal structure by virtue of its geometry and the atom positions within.
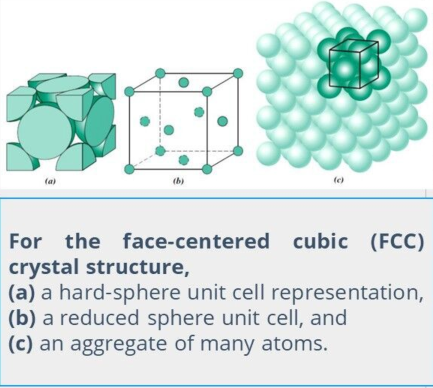
Face-Centered Cubic Crystal Structure
The crystal structure found for many metals has a unit cell of cubic geometry, with atoms located at each of the corners and the centers of all the cube faces.
These spheres or ion cores touch one another across a face diagonal; the cube edge length (a) and the atomic radius (R) are related.
Examples:
copper
aluminum
silver
gold
a = 2R√2
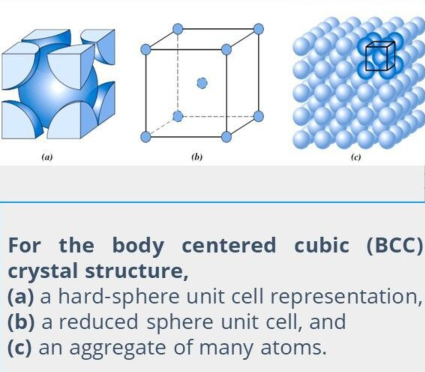
Body Centered Cubic Crystal Structure (BCC)
Another common metallic crystal structure has a cubic unit cell
atoms — located at all eight corners
a single atom — at the cube center.
Center and corner atoms touch one another along the cube diagonals, and unit cell length (a) and atomic radius (R) are related through.
a = 4R / √3
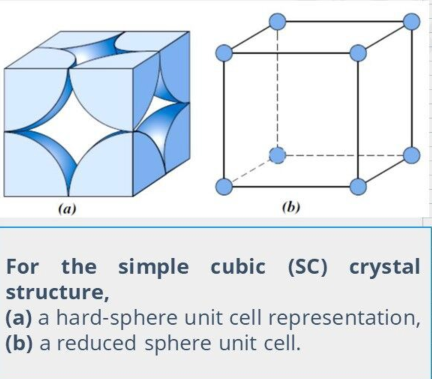
Simple Cubic
It is possible to have a unit cell that consists of atoms situated only in the corners of a tube.
None of the metallic elements have this crystal structure because of its relatively low APF.
The only — cubic element is polonium, which is considered a metalloid.
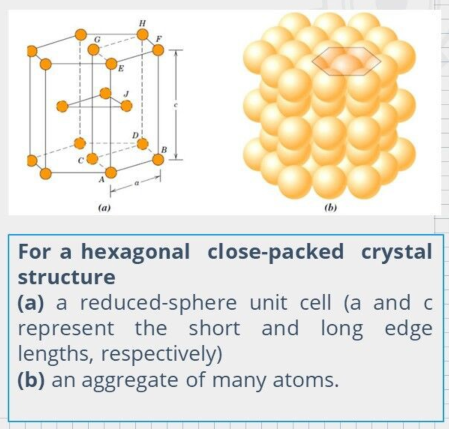
Hexagonal Close-Packed Crystal Structure (HCP)
The top and bottom faces of the unit cell consist of six atoms that form regular hexagons and surround a single atom in the center.
Crystal Systems
There are seven different possible combinations of a, b, and c; and a, b, and g, each of which represents a distinct crystal system.
These seven crystal systems are:
cubic
tetragonal
hexagonal
triclinic
orthorhombic
rhombohedral
monoclinic
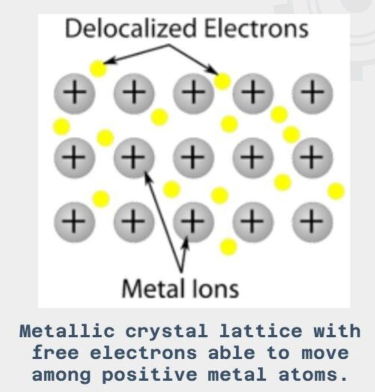
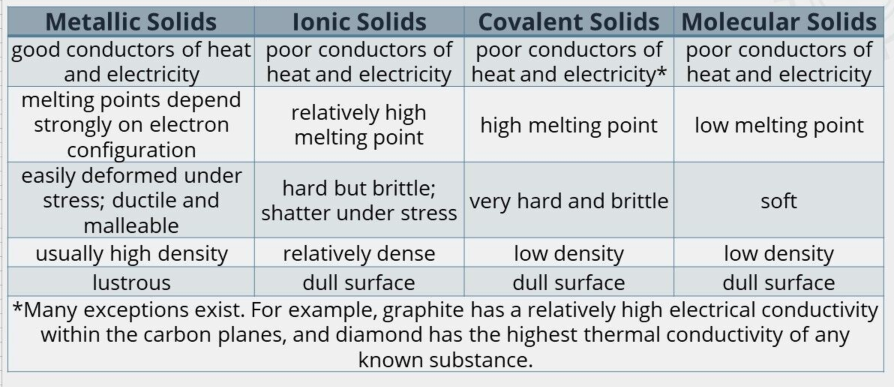
Metallic Crystals
atomic bonding is metallic.
non-directional in nature.
minimal restrictions as to the number and position of the nearest-neighbor atoms leading to relatively large numbers of nearest neighbors and dense atomic packings.
Metallic Crystals
very high melting and boiling point.
conduct both electric currents and thermal energy (heat).
ductile - capable of being ‘stretched out’ to form thin wires.
malleable - capable of being ‘hammered out’ to form thin sheets.
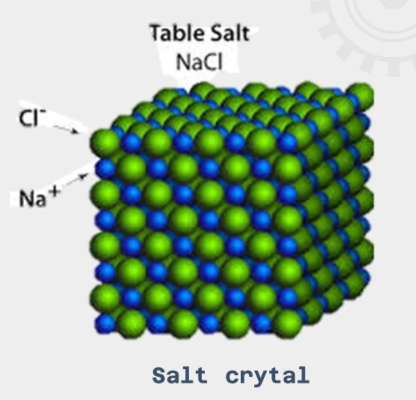
Ionic Crystals
crystal structures in which the atoms are held together through ionic bonds.
no free electrons in this structure, and rather the atoms are bound through electrostatic force arising from a difference in charge.
Ionic Crystals
do not conduct electricity when in the solid form.
very high melting points - this is because the (—) bonds in the lattice are very strong and require a lot of energy to break them.
very hard and brittle - this is because when under force, (—) with the same charge are forced to be close to one another and cause a huge electrostatic repulsion.
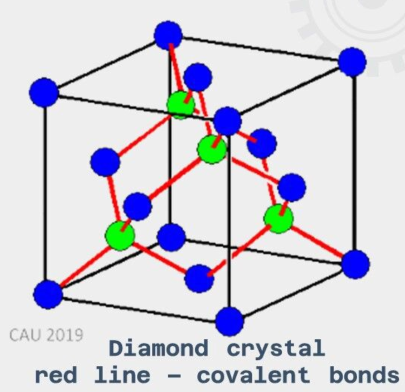
Covalent Crystals
structures in which the atoms are bonded through covalent bonds - the type of bond where the atoms share their outer electrons.
directional, which essentially forms one huge interlocking crystal structure.
Covalent Crystals
very high melting and boiling point - this bond in the lattice is incredibly strong and requires a large amount of energy to break.
don’t conduct electricity - there are no free electrons in the lattice, and so no charge carriers (graphite is the only exception to this rule, which can conduct electricity).
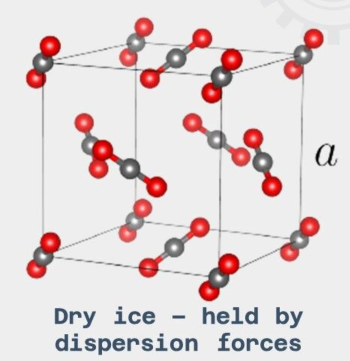
Molecular Crystals
structure in which the atoms are bonded through weak intermolecular forces.
if the structure is formed of nonpolar crystals, then the forces will be dispersion forces.
if the structure is formed of polar crystals, then the forces will be dipole-dipole.
Molecular Crystals
Low melting and boiling points - the intermolecular bonds between the atoms are fairly weak and take little energy break.
Poor at conducting electricity - there are no free electrons to act as charge carriers throughout the lattice.
Types of Crystals
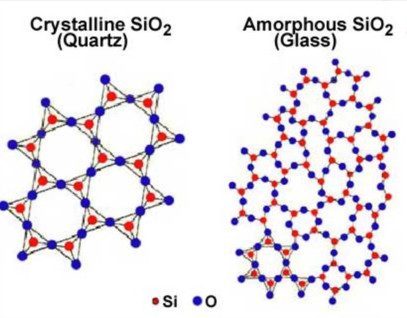
Glass
The most common example of an amorphous solid.
It lacks a regular three-dimensional arrangement of atoms.
A material made of natural products.
An inorganic solid material.
Can withstand high temperatures.
100% recyclable.
Sand
the common form of silica, easily available in nature.
the main component of glass along with the chief component silicon dioxide (SiO2).