Shit to memorise
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
NEUTRALISATION - acid + base
salt + water
NEUTRALISATION - acid + metal hydroxide
salt + water
NEUTRALISATION - acid + metal oxide
salt + water
NEUTRALISATION - acid + metal carbonate
salt + CO2 + H2O
NEUTRALISATION - acid + metal
salt + hydrogen gas
METALS - metal + oxygen
metal oxide
METALS - metal + water
metal hydroxide + hydrogen gas
METALS - acid + metal
salt + hydrogen gas
Carboxylic acids
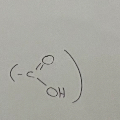
Alcohols
-OH
contain hydroxyl functional group
ends in anol
Isomer
molecules with same molecular formula but different structural formula
homologous series
a family of compounds which have the same general formula and similar chemical properties
eg: general formulas for Alkanes and Alkenes
hydrocarbon
compound which contains carbon and hydrogen only
Alkanes
CnHn2n+2
end in ane and include:
meth eth prop but pent hex hept oct non dec
only one single bond between the carbon atoms
saturated
Alkenes
CnH2n
end in ene
double bonds between the carbons atoms
unsaturated
Cycloalkanes
first member is cycloPROPANE
CnH2n
saturated
alkanes do / do not decolourise bromine water quickly
do not
alkenes do / do not decolourise water quickly
do (addition reaction)
when hydrogen is added to an alkene to make alkane this is called
hydrogenation
when water is added to an alkene to create an alcohol this is called
hydration