4- Kearns - Alkylating Agents and Antibiotics
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
What are the 3 types of cell cycle non-specific anti-cancer drugs?
alkylating agents (nitrogen mustards & nitrosoureas)- modify DNA structure
anthracyclines- abx
platinum-based compounds (platinum complexes)- modify DNA structure
think: “All Awesome Pharmacists”
Do each the following classes form covalent or intermolecular bonds?
alkylating agents (nitrogen mustards & nitrosoureas)
anthracyclines
platinum-based compounds
alkylating agents (nitrogen mustards & nitrosoureas)- COVALENT
anthracyclines- INTERMOLECULAR
platinum-based compounds- COVALENT
The efficacy of most cell-cycle nonspecific drugs are dependent on if what tumor suppressor gene is functioning?
p53
Because alkylating agents can overwhelm DNA repair mechanisms (bc of the covalent linkages) what ADR may that lead to?
2o malignancy (like leukemia)
Are most alkylating agents prodrugs? (nitrogen mustards, nitrosoureas)
yes
Do alkylating agents have a cytotoxic or cytostatic effect?
cytotoxic
What are the general ADRs of alkylating agents? (each drug class AS WELL AS drugs will also have it’s own ADRs as well but these are just common to the entire category)
BONE MARROW SUPPRESSION!!!!!!!!!!! (aka when fewer blood cells are made in the marrow)
mucosal toxicity
neurotoxicity
reproductive toxicity
avoid in cancer
What is an electrophile? What is a nucleophile? Is an alkylating agent an electrophile or nucleophile? Which is DNA?
doubt kearns will ask this but good for background
electrophile- i think of it as “electrophillic” or electron loving—> an electrophile is electron deficient so it wants to take electrons and form bonds!!!
this is the alkylating agent
nucleophile- i think of it as “nucleus loving” and it HAS electrons (electron-rich) so it wants to find a nucleus to bond to and give those electrons away
this is DNA
What type of bonds do alkylating agents form between an endogenous molecule and the alkyl group?
a. hydrogen
b. van der waals
c. covalent
d. ionic
c
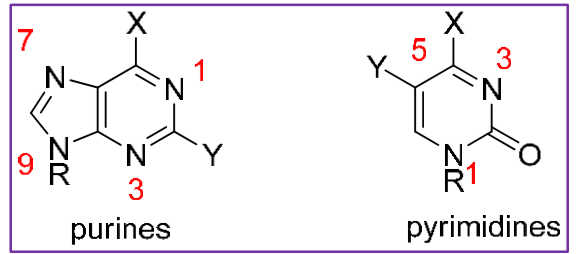
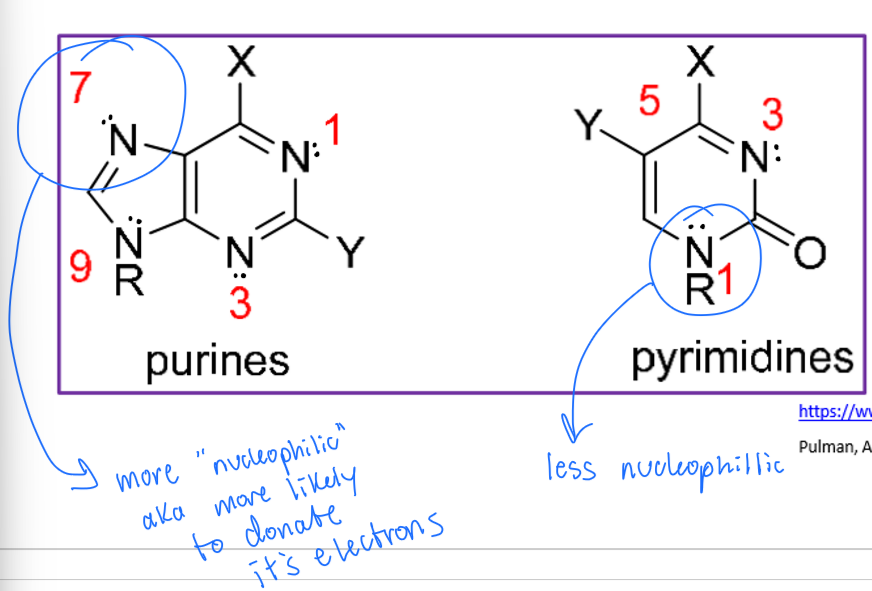
We know DNA is a nucleophile and is made of purines and pyrimidines. Does each nitrogen in DNA have the same nucleophilicity?
no! it varies. for example, the N7 on guanine is the more nucleophilic than the N1 on cytosine
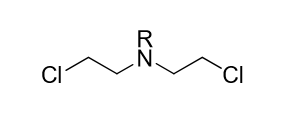
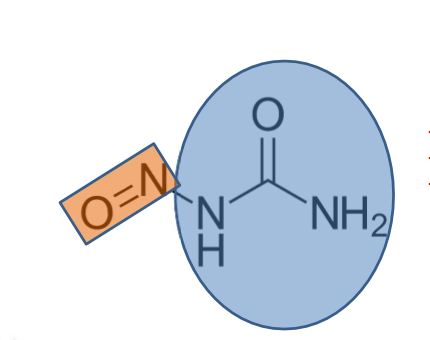
All nitrogen mustards have what functional group?
a. central amine group
b. urea group
c. transition metal electrophile
d. aromatic ring
a (pictured is an example)
(FYI: the central amine group is also called a bis-beta-haloalkylamine)
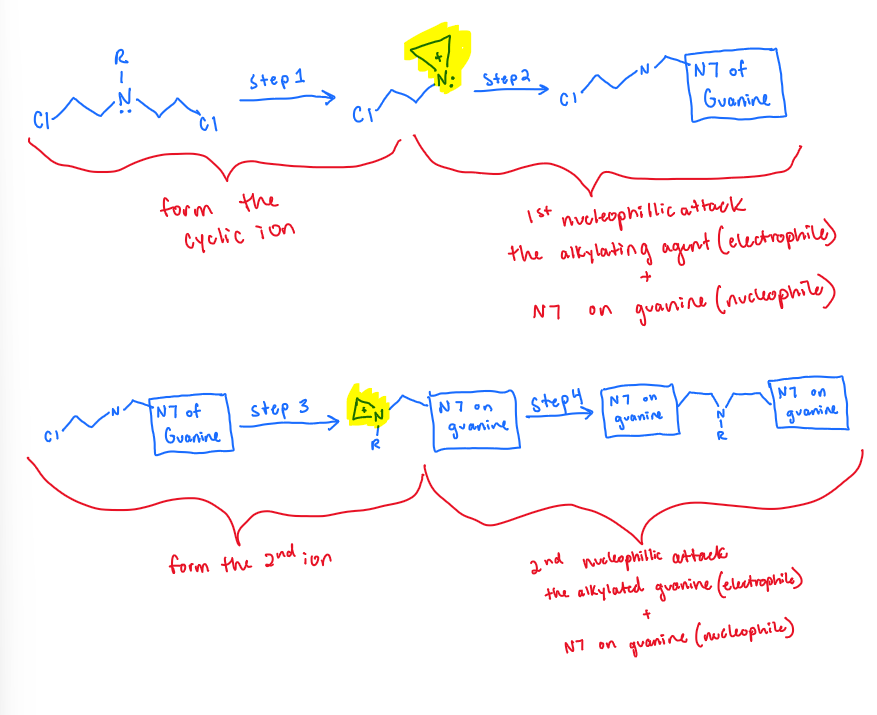
What is the goal MOA of nitrogen mustards?
DNA alkylation of GUANINE to form a cross-link
Describe the 4 step MOA of nitrogen mustards in detail:
the central amine group (bis-beta-haloalkylamine) forms cyclic ammonium ions called aziridinium ions
basically the amine group reacts with itself to form a ring
“1st nucleophile attack” —> the N7 on guanine (DNA) attacks the aziridinium ions to make an alkylated guanine
remember DNA= nucleophile, alkylating agent (in this case the azirdinium ions)= electrophile ——> these two react and form a COVALENT bond (in this case an alkylated guanine :)
a second cyclic aziridinium ion forms on the alkylated guanine
same thing as step one!!! but this time on the product from step two instead of the central amine group
“2nd nucleophile attack”—> the N7 on ANOTHER GUANINE attacks the alkylated guanine and that forms an INTERSTRAND CROSS LINK!!!!
same thing as step 2 except it’s with another guanine
SUMMARY: form a cyclic ion—> 1st nucleophile attack where guanine+ the ion form an alkylated guanine—> second cyclic ion forms —> 2nd nucleophile attack between the alkylated guanine and another guanine = RESULTS IN CROSS LINK THAT’S MESSING UP THE DNA BACKBONE!!!!!!!!!!!!!
Nitrogen mustards and nitrosoureas cause the DNA to cross link. Is this an inter or intrastrand connection?
interstrand
Nitrogen mustards CANNOT be reconstituted in _____________ or else the patient WILL DIE.
water (FYI: must be saline)
What are the class ADRs of nitrogen mustards?
(reminder each nitrogen mustards drug itself has its own additional ADRs as well)
kearns said- “know all of these”
BONE MARROW SUPPRESSION
mucosal/GI toxicity
neurotoxicity
2o malignancies (like leukemia)
reproductive toxicity
Name the drugs that are nitrogen mustards:
mechlorethamine (mustargen)
Cyclophosphamide (Cytoxan)
Ifosfamide (Ifex)
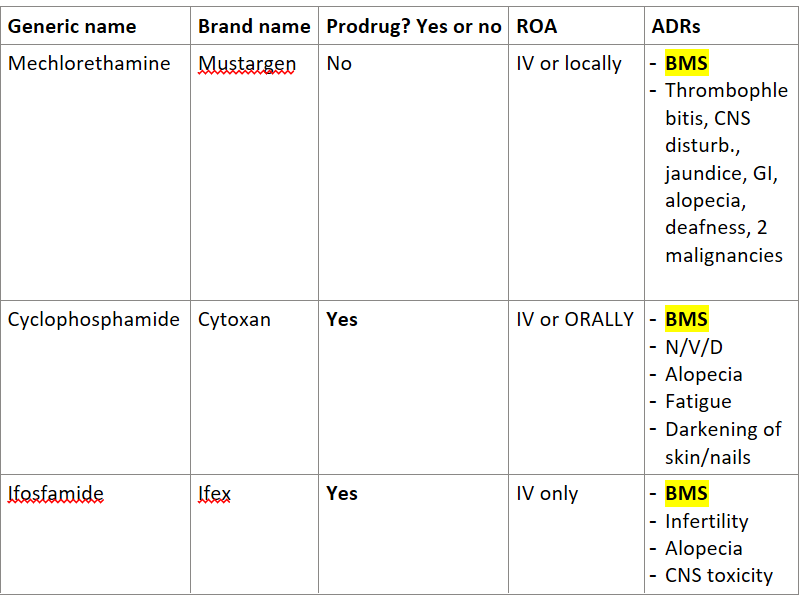
Answer the following table about nitrogen mustards:
Generic Name | Brand Name | Prodrug? Yes or No | ROA | ADRs |
mechlorethamine | ||||
Cyclophosphamide | ||||
Ifosfamide |
What is the name of the inactive metabolite formed in Ifosfamide and Cyclophosphamide?
THE INACTIVE METABOLITE IS ACROLEIN
Acrolein is a reactive __________.
a. nucleophile
b. electrophile
b
What must Cytoxan and Ifex be administered with? Why?
MESNA— In order to neutralize the reactive electrophile ACROLEIN
Compared to Cyclophosphamide, Ifosfamide has a higher risk of _____________.
hemorrhagic cystitis (inflammation/bleeding of the bladder wall)
After the reaction at the N7 position of guanine, the cell tries to fix the damage done by nitrogen mustards using what reactions?
deglycosylation
ring opening
dealkylation
What type of functional group is this? Is it lipophilic or hydrophilic?
nitrosourea- lipophillic
What is nadir? How does this apply to nitrosoureas?
nadir= refers to the lowest point that a patient's blood cell counts reach after undergoing chemotherapy
nitrosoureas cause myelosuppression (decrease in production of blood cells) and nadir is ~4 weeks
exception- steptozocin
Name the drugs that are nitrosoureas:
Carmustine (BiCNU, Gliadel)
Lomustine (CeeNU, Gleostine)
Streptozocin (Zanosar)
Unlike nitrogen mustards that form 1 electrophile, how many do nitrosoureas form?
2
Explain the MOA of Nitrosoureas in detail:
Form 2 electrophiles—> Dianzene Hydroxide (acts on DNA) and Isocyanate (acts on Proteins)
Each electrophile acts differently—> 1st nucleophilic attack
Dianzene Hydroxide—> forms chloroethyl group that will alkylate the O6 GROUP OF GUANINE
Isocyanate—> N on Lys attacks Isocyanate group
Each electrophile acts differently —> 2nd nucleophilic attack
Dianzene Hydroxide—> forms crosslink w/ DNA
Isocyanate—> leads to protein inactivation
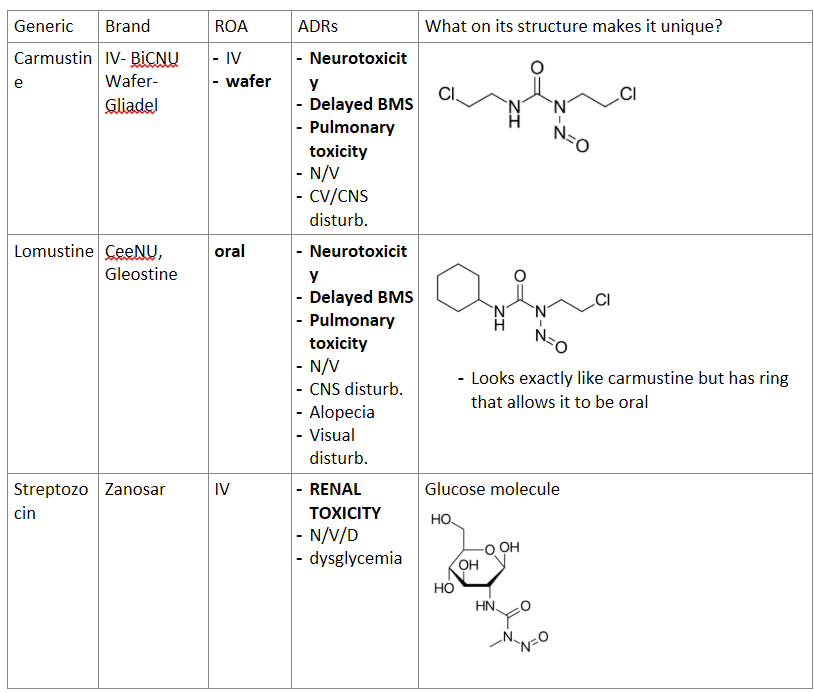
Answer the following table about nitrosoureas:
Generic | Brand | ROA | ADRs | What on its structure makes it unique? |
Carmustine |
|
|
|
|
Lomustine |
|
|
|
|
Streptozocin |
|
|
|
|
What is Streptozocin used for?
pancreatic islet cell carcinoma
What is the overall MOA of platinum complexes? (not the steps)
what do they do to DNA?
what is the result on DNA replication and protein synthesis?
what tumor suppressor protein must be functioning?
they COVALENTLY bind to nucleophilic sites on DNA
inhibits DNA replication and protein synthesis
single and double strand breaks
miscording
p53
Platinum Complexes CANNOT be administered through what kind of needles?
aluminum
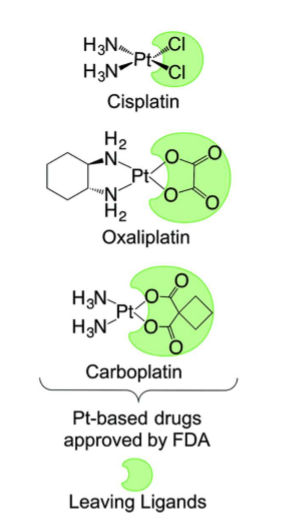
Name the drugs that are platinum complexes.
Cisplatin (Platinol)
Carboplatin (Paraplatin)
Oxaliplatin (Eloxatin)
What are the steps that platinum drug complexes take to cross link DNA?
Aquation
the ligands on each platinum complex drug are displaced by water
Form the aquo complex
+ charged molecule
aquo complex reacts with nucleophilic sites on DNA and proteins
activated platinum complexes react with other nucleophilic site OVER AND OVER to produce crosslinked DNA
Be able to recognize the structure of the platinum complex drugs and which ligands leave during step 1 of the MOA. What is similar in all of the drugs?
similar is a Pt molecule
Answer the following table about platinum complexes:
Generic | Brand | Admin | ADRs |
Cisplatin |
|
|
|
Carboplatin |
|
|
|
Oxaliplatin |
|
|
|
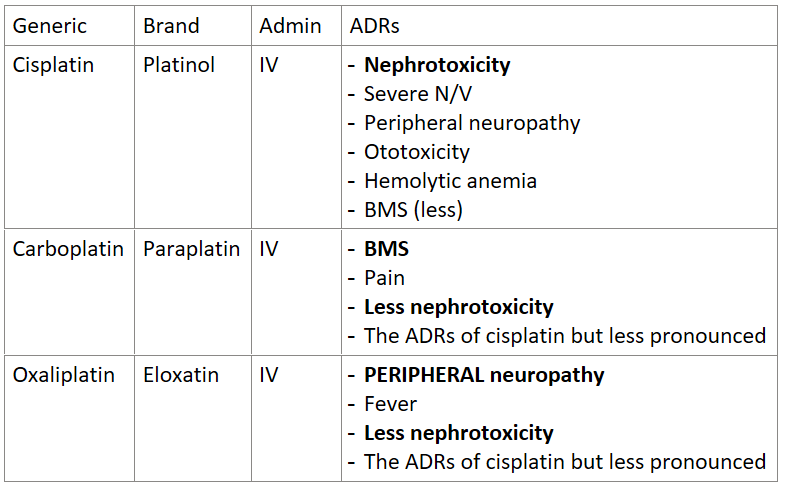
Which cell-cycle non-specific drug is photosenesitive and must be protected from light?
cisplatin
What is oxaliplatin co-administered with?
5-FU and leucovorin
How are anthracyclines different from tetracyclines?
aromaticity
What are the 2 major components of the structure of anthracyclines?
4 fused rings- A,B,C,D
sugar group attached to D ring (L-daunosamine)
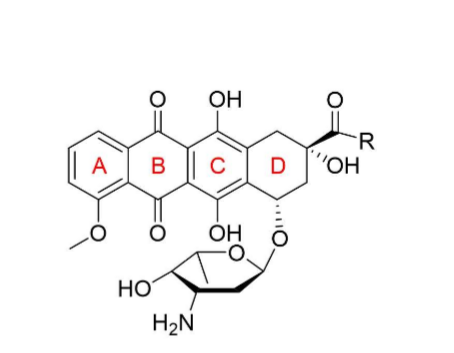
Do anthracyclines work through covalent or intermolecular bonds?
INTERMOLECULAR (VERY DIF FROM ALKYLATING AGENTS THAT USE COVALENT!!!!!!!!!!)
What specific intermolecular interaction do anthracyclines use?
pi-pi stacking
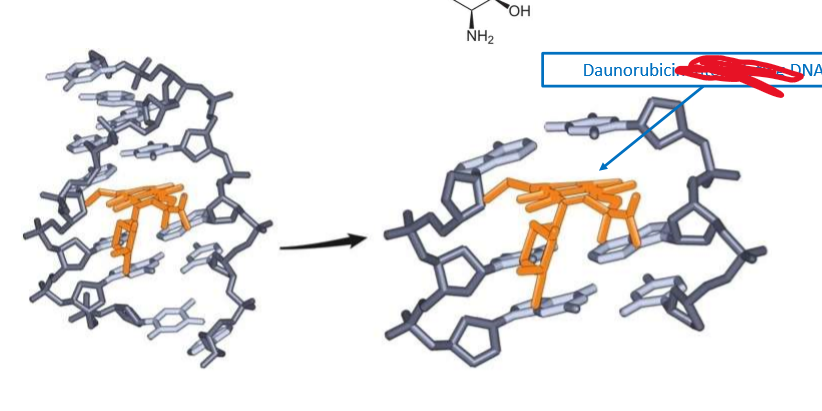
What is this picture showing?
how anthracyclines intercalate in DNA
What is the MOA of Anthracyclines?
effect on DNA structure
effect on DNA replication
generate what toxic substance?
do what to chromatin?
INTERCALATION disrupts helical structure
interferes with activity of DNA-binding enzymes
topoisomerase and polymerases
Generates FREE RADICALs = strand breaks
histone eviction—> disrupts chromatin
How are free radicals formed and neutralized in a normal healthy person? What do anthracyclines do to this pathway to treat cancer?
in a normal person—> peroxides are broken down by the enzyme CATALASE to water and oxygen!!!!!!!!!
anthracyclines—> promotes an alternative pathway called the “Fenton Pathway” to induce free radicals and cause apoptosis
Anthracyclines are given ____.
a. IV
b. oral
c. wafer
d. locally
a
For some anthracyclines, Daunorubicin and Doxorubicin a _______________formulation is available to reduce toxicity.
liposomal (FYI: liposome has hydrophillic core and hydrophobic lipid outside)
The active metabolite of anthracyclines has the ketone reduced to a ___________.
2o alcohol
What is the MAIN TOXICITY WITH ANTHRACYCLINES AND WHY?????
CARDIOTOXICITY—> this is because cardiac tissue does NOT have catalase
Bc of the toxicity associated with anthracyclines what happens with dosing?
there are lifetime dose limits
What are some other ADRs of the anthracycline class?
BMS
GI
alopecia
derm issues
What are the brand/generic names of the anthracyclines?
Daunorubicin (Caerubidine)
Doxorubicin (Adriamycin)
Epirubicin (Ellence)
Valrubicin (Valstar)
Idarubicin (Idadmycin)
Most of the Anthracyclines have a longer half-life (27-45 hrs), which anthracycline is an exception to this?
Valrubicin (t 1/2= 2hrs)
What type of drug is Bleomycin (Blenoxane)?
Fe++ chelating glycopeptide
Bleomycin is cell-cycle specific to which phase?
G2
Unlike the MOA of other anthracyclines, Bleomycin does not inhibit what enzyme?
topoisomerase
How is Bleomycin administered? How is it metabolized?
IV, IM, intrapleural administration, metabolized by hydrolase
With bleomycin what are the possible toxicities?
PULMONARY TOXICITY—> fibrosis
dermatologic toxicity
anaphylaxis reactions