Metals
1/39
Earn XP
Description and Tags
Module 3: Lecture 1
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
Metallic Bonding
final primary bonding type found in metals and their alloys
metallic materials have 1, 2, or 3 valence electrons
move through an electron cloud
remaining form ion cores = net positive charge of magnitude of # of valence
atoms in crystal structure (lattice)
good thermal and electrical conductivity
free electrons
glue that holds ion cores together
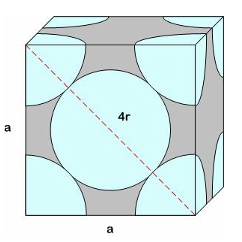
Face Centered Cubic (FCC)
atoms located at each of the corners and centers of all the cube faces
Ex) copper, silver, aluminum, gold, lead
4 atoms make up a FCC “unit cell”
Crystalline material
atoms are situated in a repeating order or periodic array over large atomic distances
convenient to subdivide the structure into small entities called “unit cells"
FCC, BCC, HCP
Body Centered Cubic (BCC)
center and corner atoms touch one another alone cube diagonals
Ex) chromium, Iron, Tungsten
2 atoms make up a BCC “unit cell”
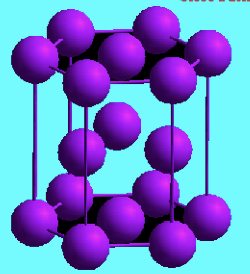
Hexagonal Close Packed (HCP)
top and bottom faces of the unit cells consist of 6 atoms in a hexagon shape that surround a single atom in the center
not symmetrical
Ex) cobalt, titanium, zinc, magnesium
6 atoms make up a HCP “unit cell”
Packing efficency
chemical, physical, and mechanical qualities, as well as a number of other attributes, are revealed by packing efficiency
FCC = 74%
BCC = 68%
Simple Cubic = 52%
Steel
an alloy composed of iron and carbon, along with additional alloying elements
amount of carbon & impurities determine the properties of the grade of steel
over 3500 grades
Alloy
material with metallic properties that is composed of 2 or more substances, of which at least one must be a metal
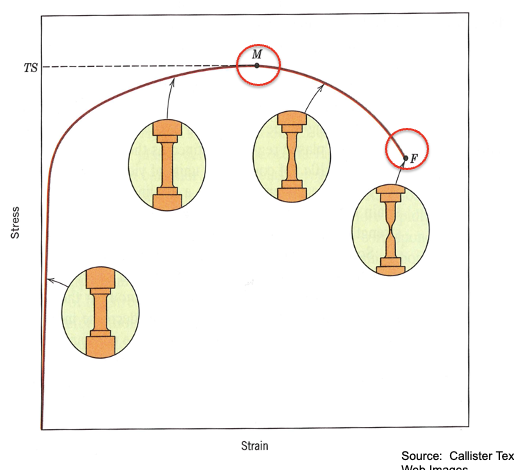
Stress-Strain behavior at fracture
F - fracture point or rupture stress
M- ultimate stress or tensile strength
elastic limit
the elastic limit is the limit beyond which the material will no longer go back to its original shape when the load is removed
proportional limit
maximum stress where the stress/strain relationship is linear elastic
elastic (Young’s) modulus
a measure of stiffness
E = stress / strain
Toughness
materials ability to absorb energy before it breaks
Modulus of toughness
area under the stress/strain curve
Ductility
amount of strain (deformation) experienced before failure
Poissons Ratio
v = -Ex / Ey
yield strength
stress at which deformations increase without an increase in load
Use the 0.2% offset method
draw line parallel to the slope of the elastic modulus, E, but offset by 0.2%
ultimate stress
maximum stress carried before failure, after this there is an increase in strain, but no increase in stress, “necking”
failure stress
stress at rupture
stress/strain curve
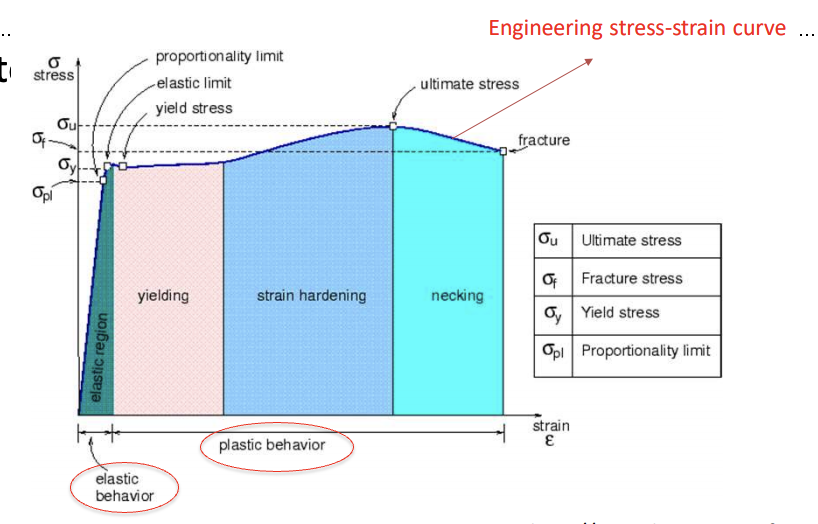
0.2% method
find 0.002 strain point on x axis
draw parallel line to the linear elastic portion of the curve
mark the intersection point of the line with the curve
this is yield stress
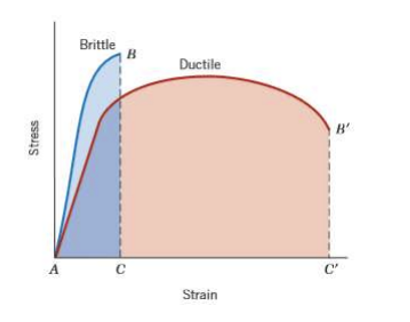
Ductility vs Toughness
Ductility - amount of strain (deformation) experienced before failure
Toughness - area under the curve, energy absorbed before failure
in the elastic region
the strain (deformation) is fully recoverable
elastic strain aka
bond stretching
plastic strain aka
bonds breaking
strain hardening
a metal is strained beyond the yield point
increasing stress is required to produce additional plastic deformation and the metal becomes stronger
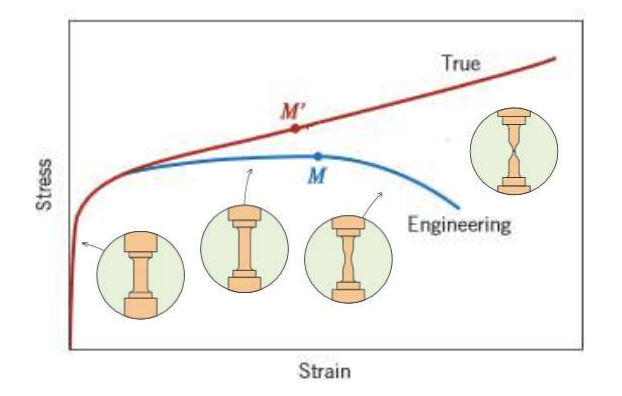
“true” vs “engineering” stress strain curve
“true” - area decreases with loading, so stress increases
“engineering” - constant area assumed
Iron-based Alloys
wrought iron - very low carbon steel, less than 0.1% C by mass
steel - 0.01 t0 1% carbon, manganese, phosphorous, sulfur, silicon, other alloys
Rebar grades
deformed or plain bars are produced in four principal (minimum yield levels)
40,000 psi Grade 40
50,000 psi Grade 50
60,000 psi Grade 60
75,000 psi grade 75
increasing steel strength
decreases the ductility
Role of carbon in steel
attracts iron alloy, ferrous alloys
If C < 1.7% by mass, carbon steel
If C > 1.7% by mass, cast IRON
limitations of ferrous alloys
relatively high densities & versatile
generally poor corrosion resistance
ferrous alloys THINK STEEL!!!
epoxy coated steel reinforcement
delays or prevents corrosion due to chlorides
the coating should be free of breaks and defects
(galvanized) steel reinforcement
zinc coated
zinc layer corrodes, so steel is protected
good for concrete subjected to carbonation
stainless steel
a minimum of 10.5% chromium and a maximum of 1.2% carbon content
nickel and molybdenum additions
Nomenclature for steels
xx is wt% C x 100
Ex) 1060 steel — plain carbon steel with 0.60 wt% C
annealing
heat treatment method where a metal is heated to a specific temp, held at that temp, and then air cooled
increases ductility and decreases hardness
more workable
quenching
process of cooling a metal at a rapid rate, martensite forms
harder metal
tempering
heating steel below the lower critical temperature
increase toughness