Acid-base indicators
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
What is an acid-base indicator?
a weak acid or weak base where the components of the conjugate acid-base pair have different colours
i.e. the acidic form differs in colour to the basic form, so the chemical changes colour at different pH valuse
What is the weak acid indicator equation?
Hln(aq) + H2O(l) ←> ln-(aq) + H3O+
color A color B
What is the weak base indicator equation?
BOH(aq) ←> B+(aq) + OH-(aq)
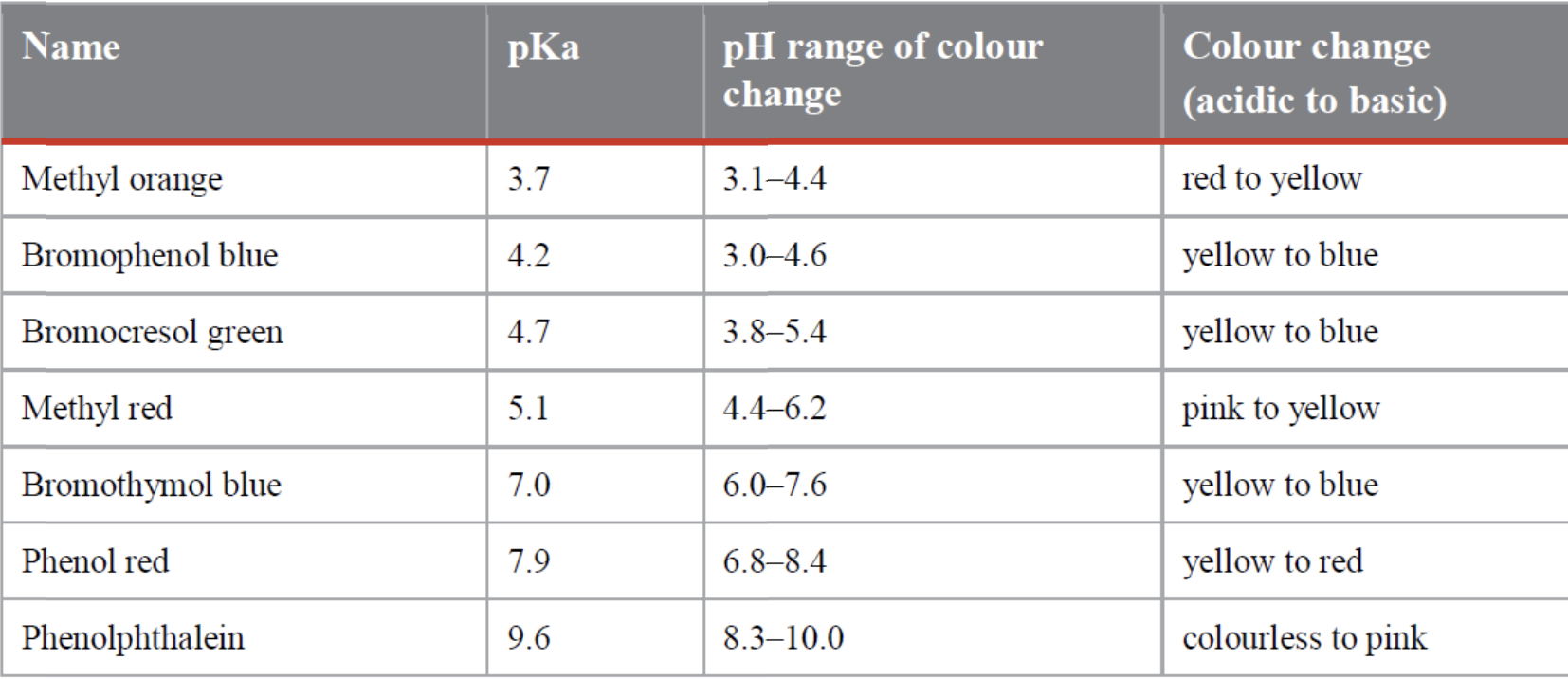
What would the appropriate indicators for titrations with equivalence points 4.5 and 5.6
4.5 = Bromocresol green. Its pKa of 4.7 is close to the equivalence point of 4.5, and 4.5 lies in the range of 3.8 – 5.4 when the indicator changes colour.
5.6 = Methyl red. Its pKa of 5.1 is close to the equivalence point of 5.6, and 5.6 lies in the range of 4.4 – 6.2 when the indicator changes
How do you know the useful pH range of an acid-base indicator?
The indicator will change colour at a pH when pH = pKa, and there is a +-1 range for when indicators tend to change colour
e.g pKa of 6.2 will change colour at a pH of 6.2, so therefore the range will be 5.2-7.2
Why do indicators change colour when pH=pKa using equations?
Hln(aq) + H2O(l) ←> ln-(aq) + H3O+
When you apply the equilibrium law to the equation, the following is true;
Ka = (H+)(ln-)/(Hln)
at equilibrium (ln-)=(Hln), therefore you can cancel them from the above equation
=(H+) → which leads to Ka=(H+) and pKa=pH