Innovation Systems and Social Values
1/10
Earn XP
Description and Tags
Panofsky: 10/13
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Vannevar Bush, MIT Engineer, and Harley Kilgore, a WV Senator had conflicting views on the ways by which science should be funded. Based on the current way science is funded currently, who won?
A) Bush (the scientist)
B) Kilgore (the politician)
C) Both achieved their goals
D) Neither achieved his goal entirely
D) Neither achieved his goal entirely
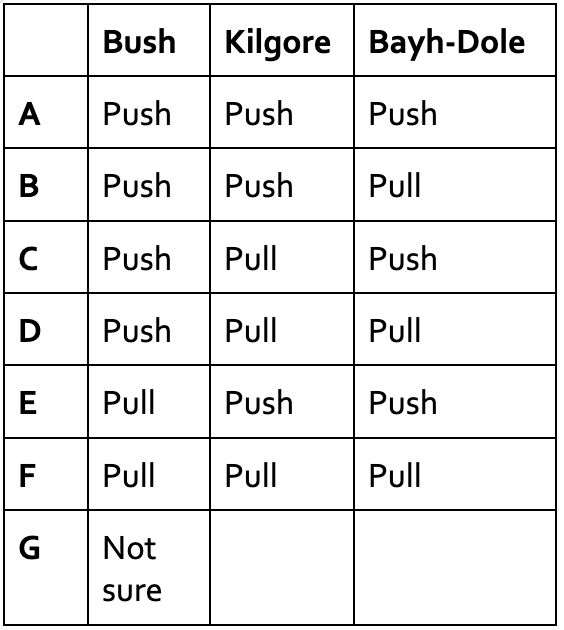
How would you classify the positions of Bush, Kilgore, and the Bayh-Dole Act based on “push and pull” factors?
B) Push, push, pull
What is an innovation system, according to Sampat and Shadlen?
A) A set of incentives, institutions, regulations, laws, actors (at multiple scales), knowledge, that societies combine to generate new technologies
B) The public systems working to develop technologies to combat the pandemic
C) The private pharmaceutical companies researching the vaccine
D) The nongovernmental organizations and philanthropies investing in therapeutics and vaccine research
A) A set of incentives, institutions, regulations, laws, actors (at multiple scales), knowledge, that societies combine to generate new technologies
Which of the following would be considered a “push” approach to biomedical innovation, based on Sampat and Shadlen’s definition?
A) Advance purchasing contracts with individual countries
B) A research grant from the NIH
C) Patents for new innovations that limit competition
D) Advance market commitments
B) A research grant from the NIH
Which of the following would be considered a “pull” approach to biomedical innovation, based on Sampat and Shadlen’s definition?
A) The Bill and Melinda Gates Foundation’s $1.2B in funding of a portfolio of COVID-19 vaccine research initiatives, called CEPI
B) Individual investors supporting multiple private research and development of vaccines
C) Facilitating collaboration between private companies
D) COVAX, or the pooled advance market commitment to procure vaccine doses to provide to 20% of populations in low-and middle-income countries.
D) COVAX, or the pooled advance market commitment to procure vaccine doses to provide to 20% of populations in low-and middle-income countries.
Which of the following is not one of the shortcomings or problems Sampat and Shadlen identified due to the rapid development of COVID-19 vaccines?
A) Supply shortages of remdesivir and antibody therapies
B) The lack of global coordination in the way we developed vaccines and ran clinical trials
C) The inability to scale up production of leading vaccines in developing nations due to storage issues and difficulties with distribution
D) The lack of coordination in funding a diverse range of potential therapeutics
E) None of the above. All of these are shortcomings the authors discussed.
E) None of the above. All of these are shortcomings the authors discussed.
Which of the following descriptions best captures “Operation Warp Speed”, based on the article by Kapczynski, Ramachandran, and Morten?
A) A “triumph and validation of industrial policy”
B) A federal initiative that provided billions in R&D subsidies to support and direct vaccine research, development and manufacturing, but failed to expand global and long-term access to the vaccines.
C) A senate initiative to leverage the Bayh-Dole Act to allow the government to license vaccine innovation to manufacture and distribute vaccines.
D) A nongovernmental organization initiative to prepare the world for the next pandemic.
B) A federal initiative that provided billions in R&D subsidies to support and direct vaccine research, development and manufacturing, but failed to expand global and long-term access to the vaccines.
The creation of the Bayh-Doyle Act of 1980 allowed for what?
A) The establishment of a National Science Foundation
B) Federal contractors and grantees would be allowed ownership of patents from federally sponsored research
C) Increase in only applied science research funding
D) Increase in only pure science research funding
B) Federal contractors and grantees would be allowed ownership of patents from federally sponsored research
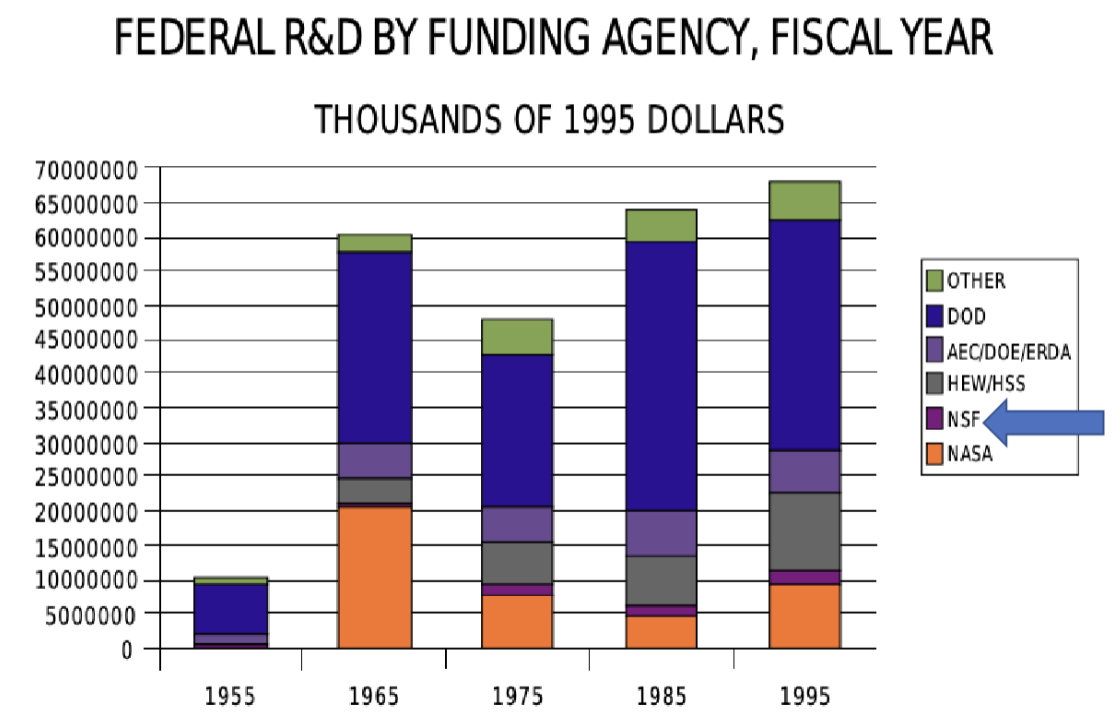
Does the above graph represent a centralized or decentralized model of federal science spending? How can you tell? Does this version of science funding satisfy either Bush or Kilgore’s goals? Explain.
The above graph represents a decentralized model of federal science spending because there are several agencies that fund scientific research, and the National Science Foundation is only a small contributor. Mission directed agencies fund most of the research. This version of science funding does not completely satisfy the goals of their Bush or Kilgore because while an NSF was established like Bush wanted, there was also funding for a mix of pure, directed, applied, and social sciences like Kilgore wanted. Bush did not get to have scientists make decisions, and Kilgore did not get democratic control over funding.
How were incentives structured in the medicine innovation system before the pandemic and how did it change during the pandemic? How did this system vary around the world?
Before the pandemic, the medicine innovation system incentivized the development of new therapeutics through “push” funding as up front research funding and “pull” incentives as patents and advance market commitments. After the pandemic, funders and incentives prioritized vaccines over therapeutics, and focused on downstream challenges such as manufacturing capacity, and late-stage clinical trials, in the US, and globally. Pull incentives also shifted to incentivize vaccine manufacturing through advance market commitments, such as COVAX, which aimed to acquire enough doses to provide vaccines to 20% of populations in low- to middle-income countries. The pandemic also highlighted the need to reconsider how the patent incentive limits access to medicines, encouraging the US government to deploy “march-in” rights via the Bayh-Dole Act to encourage generic, and more price-accessible, competition. Globally, there has also been a push to share technology and research, such as through the voluntary COVID-19 Technology Access Pool (C-TAP) effort run by the WHO.
What is Kapczynski and colleagues' main critique of how OWS was undertaken? How does OWS fail to deliver on many of its promises and leave us vulnerable to the next pandemic? What would a better industrial policy look like according to the authors?
Kapczynski, Ramachandran and Morten criticized the idea that OWS represented a paradigm shift in industrial policy-making, as it reinforced supply-side liberalism. This directed vast amounts of public funding toward private-sector vaccine research and development in the public interest, leaving control over policy and authority to them as well. OWS provided Pfizer and Moderna billions in taxpayer funding to finance their research, clinical development, manufacturing, and distribution of their vaccines. Instead of leveraging the immense public investment to enforce policies to price the vaccines accessibly and share their data and intellectual property with other researchers, OWS gave the companies patents for the vaccines, allowing them exclusive control over their pricing and distribution. This allowed the companies to “rewrite history” and mark up the price of their products, leaving many without access to the vaccine. By not leveraging their role in funding this research, the government also failed to support preparations for the next pandemic, by allowing private companies to keep their research secret, and not build capacity to collaborate on research to expedite innovations. The authors envision a better industrial policy to mandate collaboration and sharing of data to expedite research and development of new therapeutics, and build our own public R&D & manufacturing infrastructure to serve public interests instead of funnelling it directly to private companies. Essentially, public funding should support public knowledge, capacity, infrastructure, and power.