Chem 115 Exam 2
1/114
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
115 Terms
atomic mass
Is listed below the symbol of each element on the periodic table
Gives the mass of an “average” atom of each element compared to C-12
Is not the same as the mass number
Takes into account the percent of abundance of each isotope for a particular atom
molecular masses from chemical formulas
Molecular mass = sum of atomic masses
Ex. H2O
(2 x atomic mass of H) + (1 x atomic mass of O)
= (2 x 1.008 amu) + (1 x 16.00 amu)
= 18.02
By convention, we read masses off the periodic table to 4 significant figures
For ionic compounds we refer to a formula mass since ionic compounds do not consist of molecules
What is the molecular mass of cocaine, C17H21NO4?
C (17): (17)(12.011) = 204.17 amu
H (21): (21)(1.0080) = 21.168 amu
N (1): (1)(14.008) = 14.008 amu
O (4): (4)(16.000) = 64.000 amu
Add all together
= 303.35 amu
What is the formula mass of FeCl3?
Fe (1): (1)(19.00) = 19.00 amu
Cl (3): (3)(35.457) = 106.371 amu
= 125.4 amu
collection terms
States a specific number of items
1 dozen donuts = 12 donuts
1 ream of paper = 500 sheets
1 case = 24 cans
a mole of atoms
A collection that contains the same number of particles as there are carbon atoms in 12.0 g of 12C
So 1 mol of anything = 6.022x10^23 particles
Particles can be atoms, molecules, or formula units
Avogadro’s number (NA) = 6.022x10^23 particles/mol
a mole of a compund
Of a covalent compound has avogadro’s number of molecules
1 mole of CO2 = 6.022x10^23 CO2 molecules
1 mole of H2O = 6.02x10^23 H2O molecules
Of an ionic compound contains avogadro’s number of formula units
1 mole of NaCl = 6.022x10^23 NaCl formula units
1 mole of K2SO4 = 6.022x10^23 K2SO4 formula units
using avogadro’s number
Avogadro’s number, NA, is measured as 6.022x10^23
Microscale → measure chemicals in terms of atoms, molecules, and f.u. (atomic scale)
Macroscale → measure chemicals in terms of moles or mass (g, lb, kg etc) (lab scale)
Use avogadro’s number whenever converting from/to macroscale ← → microscale
Use molar mass whenever converting from/to moles ← → mass
using avogadros number cont.
Counting formula units by moles is no different than counting eggs by the dozen (12 eggs)
Since the individual particle is very small, the mole is a more practical quantity
It is a group, in which 6.022x10^23 individuals comprise 1 mole
how many moles is 6.091x10^32 molecules of water
1 mol H2O = 6.022x10^23 molecules H2O
6.091x10^32 molecules H2O | 1 mol H2O
—-------------------------------------------------------- = 1.011x10^9 mol H2O
| 6.022x10^23 molecules
how many molecules in 0.75 mol N2O5
1 mol N2O5 = 6.022x10^23 molecules N2O5
0.75 mol N2O5 | 6.022x10^23
—-------------------------------------------------- = 4.5x10^23 molecules
| 1 mol N2O5
molar mass
The mass of one mole of a substance
Is numerically equal to the atomic mass (or formula/molecular mass)
The atomic mass of an element expressed in grams
The atomic masses (found on the periodic table) provide a moles per gram conversion factor
what is the mass of 5.2 mol beryllium
1 mol Be = 9.01g Be
5.2 mol Be | 9.01g Be
—----------------------------- = 47g Be
| 1 mol Be
how many moles is 211g molybdenum
1 mol Mo = 95.94g
211g Mo | 1 mol
—------------------------------ = 2.20 mol Mo
| 95.94g
how many formula units are present in 10. mg of (NH4)2CO3
Convert 10. mg to g first → 0.010 g (NH4)2CO3
Find molar mass of (NH4)2CO3
N: (2)(14.01) = 28.01 g/mol
H: (8)(1.01) = 8.08 g/mol
C: (1)(12.01) = 12.01 g/mol
O: (3)(16.00) = 48.00 g/mol
= 96.11 g/mol (molar mass of entire thing) = 1 mol (NH4)2CO3
how many formula units are present in 10. mg of (NH4)2CO3 cont.
0.010 g (NH4)2CO3 | 1 mol (NH4)2CO3 | 6.022x10^23 FU (NH4)2CO3
—--------------------------------------------------------------------------------------
| 96.11 g/mol | 1 mol
= 6.3x10^19 FU (NH4)2CO3
how many ions are present
NH + NH4 + CO3 -
3 ions per fu
= 6.3x10^19 x 3 = 1.9x10^20 total ions
using the chemical formula
Subscripts within chemical formula can be used as conversion factors
Relate components of a compound to the compound quantity we look at the chemical formula
In Na2CO3 there are 3 relationships
2 mol Na: 1 mol Na2CO3
1 mol C: 1 mol Na2CO3
3 mol O: 1 mol Na2CO3
We can also use these on the atomic scale
1 atom C: 1 fu Na2CO3
In 0.347 mol Cl2O7, how many atoms of oxygen are present?
1 mol = 6.022x10^23
0.347 mol CL2O7 | 7 mol O | 6.022x10^23 atoms
—---------------------------------------------------------------------------
| 1 mol Cl2O7 | 1 mol O
= 1.46x10^24 O atoms
How many moles of calcium are combined with 7.67 mol N in Ca3N2
1 mol = 6.022x10^23
Ca3N2 = 3 Ca for every 2 N
7.67 mol N | 3 mol Ca
—------------------------------------ = 11.5 mol Ca
| 2 mol N
How many moles is 2.573 g tetraphosphorus decaoxide?
Find molar mass:
P = 30.97(4) = 123.88 g/mol
O = 16(10) = 160 g/mol
Total = 123.88 + 160 = 283.9 g/mol
2.573 g P4O10 | 1 mol
—---------------------------------- = 0.009063 mol P4O10
| 283.9 g
What is the mass (in g) of 1 molecule of P4O10 (give answer in 4 sf)
Molar mass - 283.9 g/mol
1 mol = 6.022x10^23
283.9 g P4O10 | 1 mol P4O10
—-----------------------------------------------------------------
1 mol P4O10 | 6.022x10^23 molecules P4O10
= 4.714x10^-22 g/molecule P4O10
In a sample of Fe3O4 there are 11.7 g of oxygen. What mass of iron is combined with the oxygen?
For every 3 Fe, theres 4 O
Atomic mass Fe = 55.85 g/mol
Atomic mass O = 16.00 g/mol
11.7 g O | 1 mol O | 3 mol Fe | 55.85 g Fe
—-------------------------------------------------------------- = 30.6 g Fe
| 16.00 g O | 4 mol O | 1 mol Fe
percent composition
Is a list of the mass percent of each element in a compound
Na2CO3 is
43.38% Na
11.33% C
45.29% O
percent composition cont.
How is it calculated? What is the % C in CO2?
Determine the molar mass of the compound
Molar mass of CO2 = 44.0 g/mol
Multiply the ratio mass of the element to the molar mass of the compound by 100
(12.0107)(44.0) x 100 = 27.3 %C
((2)(15.9994)/44.0) x 100 = 72.7 %O
theoretical weight/mass percent
Ex. in a lab, a student attempted to prepare the compound iron (III) chloride, the student sent a small portion of the sample away for chemical analysis, the lab returned the following results of percentages of each element in the compound
Element Exp. %
Fe 34.0%
Cl 66.0%
theoretical weight/mass percent cont.
Did the student prepare iron(III) chloride? To decide, calculate the theoretical mass percent of each element in the compound
Theoretical mass percent = mass of element in 1 mol
—---------------------------------- x100
Mass of 1 mol of compound
Calculate the theoretical mass percent of carbon in LSD (C20H25N3O)
Mass of 1 mol C = 12.01 g
C = 12.01(20) = 240.2
H = 1.01(25) = 25.25
N = 14.01(3) = 42.03
O = 16.00(1) = 16.00
Total molar mass of LSD = 323.5 g/mol
tm % of carbon = 240.2
—--------- x 100 = 74.3% C
323.5
chemical formulas: molecular vs empirical
Molecular formula - chemical formula with subscripts that give actual whole # ratio of elements in a compound, is a whole # multiple of the empirical formula
Empirical formula - chemical formula with subscripts that give the smallest whole # ratio of elements in a compound
Compound Molec. Formula Emp. Formula
Acetylene C2H2 CH
Ethane C2H6 CH3
Benzene C6H6 CH
Butane C4H10 C2H5
Note: ionic compounds only have empirical formulas due to the definition of the formula unit
calculation of empirical formula from wt% and mass data
Step 1: find masses of all elements present
Step 2: convert masses to moles
Step 3: divide by smallest 3 moles found to obtain mole ratios
Step 4: if mole ratios are close enough to whole numbers, round, and use these as subscripts in empirical formula
Step 5: if mole ratios are not close enough to whole numbers, multiply by a small whole # to obtain whole numbers
wt% data
In solution, a student reacted with ruthenium(III) chloride and lithium sulfide . The black precipitate obtained was heated in H2S. After heating, the black precipitate was found to contain 61.18% Ru and 38.82%S. What was the empirical formula of the black precipitate?
Write what you know
Ru: 61.18%
S: 38.82%
Step 1
Assume theres 1g of compound
Mass Ru = 0.6118g
Mass S = 0.3882
Step 2
0.6118g Ru | 1 mol
—------------------------------- = 0.0060514 mol Ru
| 101.1g Ru
0.3882g S | 1 mol
—------------------------------- = 0.012105 mol S
| 32.07g S
wt% data cont
Step 3
Mol Ru = 0.0060514
—-------------- = 1
0.0060514
Mol S = 0.012105
—-------------- = about 2
0.0060514
Step 4
Ru1S2
= RuS2
Mass data
A 0.8684 g sample of sodium pertechnetate was analyzed and found to contain 0.1114 g Na, 0.4562 g Tc and oxygen. What is the empirical formula of sodium pertechnetate?
Step 1
Na: 0.114g
Tc: 0.4562g
O: (0.8684 - 0.114 - 0.4562) = 0.3008g
Step 2
Na:0.0048456 mol
Tc: 0.0046551 mol
O: 0.0188 mol
mass data cont.
Step 3
Tc has the smallest number
Na = 0.0048456/0.0046551 = 1
Tc = 0.0046551/0.0046551 = 1
O = 0.0188/0.0046551 = 4
Step 4
Na1Tc1O4
= NaTcO4
When you do not get whole numbers
Determine the empirical formula for a 100.0 g sample of phosphorus and oxygen that was found to contain 43.7 g of phosphorus
Step 1
P: 43.7g
O: 100.00 - 43.7 = 56.3g
Step 2
P: 1.4110 mol
O: 3.5188 mol
Step 3
P: 1.4110/1.4110 = 1
O: 3.5188/1.4110 = 2.49 (cannot round up or down)
when you do not get whole numbers cont.
Skip step 4
Step 5
2.49 is close to 2.5
2.49 x 2 = about 5
P: 1 x 2 = 2
O: 2.49 x 2 = 5
= P2O5
empirical formulas from combustion reaction data
Step 1: calculate mass from CO2 produced
Step 2: calculate mass H from H2O produced
Step 3: calculate mass “O” from law of conservation of mass
Step 4: determine the empirical formula
A 5.048 g sample of a compound containing only C, H, and O was placed in a combustion furnace. Combustion of the sample yielded 7.406 g CO2 and 3.027 g H2O. calculate the empirical formula of this compound
Step 1
5.048g sample, after combustion produced 7.406g CO2 and 3.027g H2O
7.406g CO2 | 1 mol | 1 mol C | 12.01g C
—-------------------------------------------------------------------------- = 2.0210g C
| 44.01g CO2 | 1 mol CO2 | 1 mol C
Step 2
3.027g H2O | 1 mol | 2 mol H | 1.01g H
—-------------------------------------------------------------------------- = 0.33951g H
| 18.01g H2O | 1 mol H2O | 1 mol H
combustion empirical formula cont.
Step 3
Mass O = 5.048g - 2.0210 - 0.33951 = 2.6985g O
Step 4
Moles C: 0.1683 mol C (smallest #)
Moles H: 0.3361 mol H
Moles O: 0.1687 mol O
C: 0.1683/0.1683 = 1
H: 0.3361/0.1683 = 2
O: 0.16873/0.1683 = 1
C1H2O
= CH2O
obtaining the molecular formula
Two pieces of info are needed
empirical formula
Molar mass
Remember - molecular formula is whole # multiple of empirical formula
Ex. CxHy (x n) = CnxHny
To find the molecular formula, it is necessary to find the whole #
Whole # = molar mass
—----------------
Empirical mass
A compound has an empirical formula of C10H21 and a molar mass of 282.54. What is the molecular formula of this compound?
C10H21 - empirical formula
282.54 - molar mass
282.54
—--------- = about 2
141.3
= C20H42
chemical equations
A shorthand way of describing a chemical change or reaction
Reactants → products
All chemical equations must be balanced
Material balance - number of atoms of each element must be the same on both sides of the equation
Ex. CH4 + 2 O2 → CO2 + H2O
Charge balance - net charge on reactant side must be equal to net charge on product side
Ex. H3PO4 + 3 H2O → PO4 -3 + 3 H3O +1
balancing chemical equations
There are no set rules
Guidelines:
Adjust the coefficients, never change the subscripts
Start with the most complicated species, or start with an element that appears in only one substance on both sides of the equation
Balance polyatomic ions as a group
By convention, write with the smallest set of whole number coefficients
balance O2 + SO2 → SO3
O: 4 O: 3
S: 1 S: 1
O2 +SO2 → 2SO3
O: 4 O: 6
S: 1 S: 2
O2 + 2SO2 → 2SO3 (balanced)
O: 6 O: 6
S: 2 S: 2
balance C4H10O + O2 → CO2 + H2O
C: 4 C: 1
H: 10 H: 2
O: 3 O: 3
C4H10O + O2 → 4CO2 + H2O
C: 4 C: 4
H: 10 H: 2
O: 3 O: 9
C4H10O + O2 → 4CO2 + 5H2O
cont.
C: 4 C: 4
H: 10 H: 10
O: 3 O: 13
C4H10O + 6O2 → 4CO2 + 5H2O (balanced)
C: 4 C: 4
H: 10 H: 10
O: 13 O: 13
balance C8H18 + O2 → CO2 + H2O
C: 8 C: 1
H: 18 H: 2
O: 2 O: 3
C8H18 + O2 → 8CO2 + H2O
C: 8 C: 8
H: 18 H: 2
O: 2 O: 17
C8H18 + O2 → 8CO2 + 9H2O
C: 8 C: 8
H: 18 H: 18
O: 2 O: 25
cont.
C8H18 + 25O2 → 8CO2 + 9H2O
C: 8 C: 8
H: 18 H: 18
O: 50 O: 25
C8H18 + 25O2 → 8CO2 + 18H2O
C: 8 C: 8
H: 18 H: 36
O: 50 O: 34
C8H18 + 25O2 → 16CO2 + 18H2O
C: 8 C: 16
H: 18 H: 36
O: 50 O: 50
2 C8H18 + 25O2 → 16CO2 + 18H2O (balanced)
C: 16 C: 16
H: 36 H: 36
O: 50 O: 50
balance Al2O3 + H2SO4 → Al2(SO4)3 + H2O
Al: 2 Al: 2
O: 3 O: 1
H: 2 H: 2
SO4: 1 SO4: 3
Al2O3 + H2SO4 → Al2(SO4)3 + 3H2O
Al: 2 Al: 2
O: 3 O: 3
H: 2 H: 6
SO4: 1 SO4: 3
Al2O3 + 3H2SO4 → Al2(SO4)3 + 3H2O (balanced)
Al: 2 Al: 2
O: 3 O: 3
H: 6 H: 6
SO4: 3 SO4: 3
stoichiometry
Use of the coefficients in a balanced equation to relate amounts of chemicals
Coefficients in balanced equation can be interpreted on two different scales
Microscale - atoms, molecules, formula units
Macroscale - moles
If 0.347 mol sulfuric acid are reacted with excess Al2O3, how many moles of aluminum sulfate can be produced?
Al2O3 + 3H2SO4 → Al2(SO4)3 + 3H2O
0.347 mol HSO4 | 1 mol Al2(SO4)3
—----------------------------------------------- = 0.116 mol Al2(SO4)3
| 3 mol H2SO4
How many moles of sulfuric acid are needed to react with 1.07 mol Al2O3?
1.07 mol Al2O3 | 3 mol H2SO4
—------------------------------------------ = 3.21 mol H2SO4
| 1 mol Al2O3
3Mg + 2NH3 → Mg3N2 + 3H2
Magnesium nitride is formed by the reaction of magnesium metal with gaseous ammonia. What mass (in g) of magnesium nitride can be produced from 10.57 g of magnesium? (the other product is gaseous hydrogen)
g Mg → mol Mg → mol Mg3N2 → g Mg3N2
MM of Mg = 24.32 g/mol
MM of Mg3N2 = 101.0 g/mol
10.57g Mg | 1 mol Mg | 1 mol Mg3N2 | 101.0g Mg3N2
—-------------------------------------------------------------------------- = 14.63 g Mg3N2
| 24.32 g Mg | 3 mol Mg | 1 mol Mg3N2
limiting reagent
Consider the reaction of N2 with H2 to form NH3
N2(g) + 3H2(g) → 2NH3(g)
The stoichiometry suggests that for every mole of N2, we will need 3 moles of H2 to form 2 moles of NH3
So what happens if these proportions are not met? The reaction proceeds, to use up one of the reactants (the limiting reagent) and not all of the other reactant (because it is in excess)
limiting reagent cont.
Why would an excess be used?
Some reactions proceed better (more to completion) in the presence of an excess of one reagent
One reagent may be expensive, use an excess of the cheaper reagent to ensure that the more costly reagent is used up and not wasted
limiting reagent problems
One or more reagents is present in an excess amount and one reagent is limiting (ie limits the amount of product formed)
Limiting reagent: reagent that limits the amount of product formed, all used up during the reaction
Excess reagent: reagent that does not limit the amount of product formed,, some of this reagent is left over after the reaction
Step 1: convert both reactants to moles of the same product
Step 2: identify the limiting reagent by comparing moles of product determined in step 1, the reactant that produces the least amount of product is the limiting reagent, the other is the excess
Step 3: use the limiting reagent to answer the problem
3 Li2S + 2 RuCl3 → Ru2S3 + 6 LiCl
If 2.0 mol RuCl3 is reacted with 5.0 mol Li2S, how many moles of Ru2S3 can be formed?
2.0 mol RuCl3 | 1 mol Ru2S3
—------------------------------------- = 1 mol Ru2S3
| 2 mol RuCl3
5.0 mol Li2S | 1 mol Ru2S3
—------------------------------------ = 1.7 mol Ru2S3
| 3 mol Li2S
The reagent that produces the smaller number of moles is the limiting reagent
RuCl3 is the limiting reagent
Li2S is the excess reagent
1 mol of RuCl3 can be formed
cont.
How many moles of which reagent are left?
2.0 mol RuCl3 | 3 mol Li2S
—------------------------------------ = 3.0 mol Li2S (mols consumed in the reaction)
| 2 mol RuCl3
Mols of Li2S left over?: 2.0 mol Li2S
If 7.0 mol hydrogen sulfide are reacted with 3.0 mol sulfur dioxide, how many moles of sulfur (S) can be formed (the other product is water)? How many moles of which reagent are left?
2H2S + SO2 → 3S + 2H2O
7.0 mol H2S | 3 mol S
—----------------------------------- = 10.5 mol S (produced)
| 2 mol H2S
3.0 mol SO2 | 3 mol S
—-------------------------------------- = 9 mol S (produced)
| 1 mol SO2
cont.
SO2 is the limiting reagent
H2S is the excess reagent
How many moles of which reagent are left?:
3.0 mol SO2 | 2 mol H2S
—----------------------------------- = 6 mol H2S (was consumed)
| 1 mol SO2
1 mol of H2S left over
percent yield
Most reactions do not proceed 100% to completion, as a result, the percent yield is less than 100%
Percent yield = actual yield
—-------------- x 100%
theoretical yield
cont.
Actual yield: amount of product obtained experimentally
theoretical yield: maximum amount of product that could be obtained based upon stoichiometric calculations (ie from amount of reagents)
Actual yield < theoretical yield due to:
Mechanical losses (product stuck to glassware, filter paper, etc)
Product evaporation
Competing reaction that uses up some of reagent to produce undesired product
Reaction proceeds less than 100% to completion
nitrogen reacts with hydrogen to form ammonia as shown, when 15g of nitrogen reacts with excess hydrogen 16.82g of ammonia was produced, what is the percent yield
N2 + 3H2 —> 2NH3
N; 28.04 g/mol
NH3: 17.04 g.mol
15g N2 | 1 mol
-------------------------- = 0.5353319 mol N2
| 28.04 g/mol
0.5353319 mol N2 | 2 mol NH3
------------------------------------------------ = 1,07066 mol NH3
| 1 mol N2
cont.
1.07 mol NH3 × 17.04 g/mol NH3 = 18.23g NH3
16.82
----------- x 100 = 92.27%
18.23
if the percent yield of NO was 84.4% and 26.9g of NO were isolated what mass of O2 was reacted
NH3: 17.04g/mol
O2: 32 g/mol
NO: 30.01 g/mol
84.4 = 26.9
------------ = 0.844t = 26.9
theoretical %
t = 31.87g NO
31.87g NO | 1 mol
------------------------------------ = 1.06197 mol NO
| 30.01 g/mol
cont.
1.06197 mol NO | 5 mol O2
-------------------------------------------- = 1.32747 mol O2
| 4 mol NO
1.32747 mol O2. | 32 g O2
------------------------------------------ = 42.5g O2
| 1 mol
solutions
Many chemical reactions take place in solution
Pb(NO3)2(s) + Kl(s): no reaction, obtain a physical mixture of two solids
Why? In solid, ions are locked in place and not free to move around and interact
Pb(NO3)2(aq) + 2 Kl(aq) → PbI2(s) + 2 KNO3(aq): reaction occurs
Why? In solution, the ions are highly mobile and are free to move around and interact (and switch partners)
solutions cont.
A solution is a homogeneous mixture in which the two or more components mix freely
The solvent is the component present in the largest amount
A solute is any substance dissolved in the solvent, the solution is named by the solute
May be characterized using a solute-to-solvent ratio called the concentration
dilute vs concentrated
The dilute solution on the left has less solute per unit volume than the (more) concentrated solution on the right
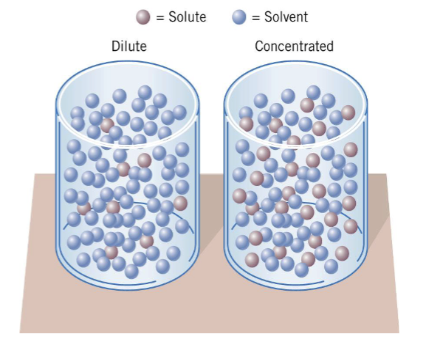
solution definitions
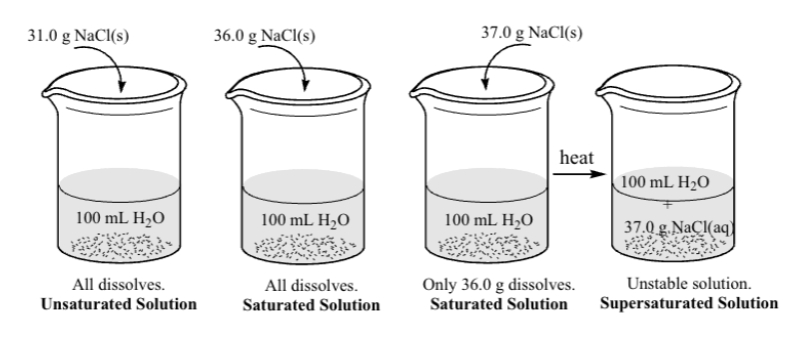
A solution is said to be saturated when no more solute can be dissolved at the current temperature
A solution containing less solute is called unsaturated because it is able to dissolve more solute
The solubility of a solute is the number of grams of solute that can dissolve in 100 grams of solvent at a given temperature: g/100mL
Solubility of NaCl = 36.0g/100mL at 20 C, a maximum of 36.0g NaCl will dissolve per 100 mL of water
molarity
Measure of amount of solute dissolved in solution
Molarity = mol solute
—--------------- = M (molar)
L of solution
If 75.3g KI are dissolved in enough water to prepare 750 mL of solution, what is the concentration of the resulting solution?
75.3g KI | 1 mol KI
—-------------------------- = 0.454 mol KI
| 166g KI
750 mL → 0.750 L of solution
Moles = molarity x volume
0.454 = M(0.750)
= 0.0605 M KI
What mass (in g) of HNO3 is needed to prepare 2.0L of 0.750 M HNO3 in solution?
Find MM HNO3
= 63.02 g/mol
0.750 mol HNO3 | 2.00 L | 63.02 g
—--------------------------------------------------- = 94.5g HNO3
1 L | 1 | 1 mol HNO3
What volume (in mL) of 0.452 M NaOH solution will provide 1.20g NaOH?
Find MM NaOH
= 40 g/mol
1.20g NaOH | 1 mol
—--------------------------- = 0.0300 mol NaOH
| 40g
Moles = molarity x volume
0.0300 = 0.452(V)
= 0.06637 L → 66.37 mL
dilution
Addition of pure solvent into a solution
Increases the solution volume
Has no affect on the number of moles M of solute
Decreases the solution concentration
(M1)(V1) = (M2)(V2)
25.00 mL of 0.750 M HNO3 is added to enough water to prepare 200. mL of solution, what is the concentration of the resulting solution?
(M1)(V1) = (M2)(V2)
(0.750)(0.2500) = (M2)(0.200)
18.75 = 0.200(M2)
0.0938 M HNO3
What volume of 5.0 M H2SO4 solution is needed to prepare 100. mL of 1.25 M H2SO4 solution?
(M1)(V1) = (M2)(V2)
(1.25)(0.100) = (5.0)(V2)
0.125 = 5.0(V2)
= 0.025 L H2SO4
molarity of ions in solution
Of greatest importance for solutions of strong electrolytes
In a 0.50 M AlCl3 solution, what is the concentration of chloride ions?
0.50 mol AlCl3 | 3 mol Cl
—--------------------------------- = 1.5 mol/L Cl = 1.5 M Cl
| 1 mol Al
How many moles of nitrate ions are present in 200. mL of 0.20 M Pb(NO3)2?
n = (M)(V)
= (0.20)(0.200)
= 0.0400 mol Pb(NO3)2
0.0400 mol Pb(NO3)2 | 2 mol NO3
—-------------------------------------------------- = 0.080 mol NO3
| 1 mol Pb(NO3)2
A solution is prepared by mixing 75.0 mL of 0.150 M AlCl3 with 75.0 mL of 0.250 M CaCl2, what is the concentration of chloride ions in the resulting solution?
Solution 1:
V = 75.0 mL = 0.075 L
M = 0.150 mol/L AlCl3
n = ?
n = (M)(V)
= (0.150)(0.075)
= 0.01125 mol AlCl3
Find mol of Cl
(0.1125)(3) = 0.03375 mol Cl
cont.
Solution 2:
V = 75.0 mL = 0.075 L
M = 0.250 mol/L CaCl2
n = ?
n = (M)(V)
= (0.250)(0.075)
= 0.01875 mol CaCl2
Find mol Cl
(0.01875)(2) = 0.0375 mol Cl
cont.
Add two mol of Cl together to get final mol amt of Cl
0.03375 + 0.0375 = 0.07125
Divide by total volume
0.075 + 0.075 = 0.15
0.07125 / 0.15 = 0.475
classification of chemical reactions
Precipitation reaction: reaction accompanied by formation of an insoluble precipitate (i.e. solid)
Driving force → formation of precipitate
Ex.
Pb(NO3)2 (aq) + K2CrO4 (aq) → PbCrO4 (s) + 2 KNO3 (aq): creates yellow precipitate
Soluble: dissolved to a significant extent in the solvent, if solubility > 0.1 M, then soluble, when solvent is water, use aqueous (aq) to designate
Insoluble: does not dissolve to a significant extent in the solvent, if solubility < 0.1 M, then insoluble, when insoluble compound is an ionic compound in water, it will precipitate
solubility rules
Allow us to predict whether an ionic compound (salt) is soluble or insoluble in water (will have to memorize)
Ionic compounds that contain group IA metal cations (Na+, K+, Li+, etc) or ammonium (NH4+) are soluble
Ionic compounds that contain NO3-, ClO3-, ClO4-, C2H3O2- or HCO3- are soluble
Ionic compounds that contain Cl-, Br- or I- are soluble except when combined with Ag+, Hg2 +2, or Pb +2
solubility rules cont.
Most compounds that contain SO4 -2 are soluble except when combined with Ag+, Hg2 +2, Pb +2, Ca +2, Sr +2, or Ba +2 (Ag2SO4 is sparingly soluble)
Most ionic compounds that contain OH-, O -2, CO3 -2, PO4 -3, CrO4 -2, or S -2 are insoluble except when combined with group IA metal cations or NH4+
Exception: Ca(OH)2, Ba(OH), and Sr(OH) are considered soluble, group IA and heavier group IIA oxides react with water to form corresponding hydroxide which is soluble
Na2O (s) + H2O → 2 NaOH (aq)
CaO (s) + H2O → Ca(OH)2 (aq)
electrolytes
When a substance is dissolved in water, the resulting solution can be classified according to its electrical conductivity
In order for a solution to conduct an electrical current, mobile ions must be present
strong electrolytes
Conducts strong electrical current in aq solution
High concentration of mobile ions in solution
Substance dissolves and dissociates/ionizes 100% into ions
Strong acids, strong bases, and soluble ionic compounds
weak electrolytes
Conducts weak electrical current in aq solution
Low concentration of mobile ions in solution
Substance dissolved but less than 100% ionizes into ions
Weak acids and weak bases
nonelectrolyte
Conducts no electrical current in aq solution
No mobile ions in solution
Substance dissolves as neutral molecules (none produces ions)
Soluble molecular compounds (C2H3OH, C12H22O11)
writing molecular equations as total equations
Since strong electrolytes exist as dissociated ions in solution, we can show this in an equation
Identify the strong electrolytes
Separate the ions in the strong electrolytes
Retain the number of ions (subscripts become coefficients)
Show the states as recorded in the molecular equations
convert to total ionic equation Pb(NO3)2 (aq) + 2KI (aq) → PbI2 (s) + 2KNO3 (aq)
Pb 2+ (aq) + 2NO3- (aq) + 2K+ (aq) + 2I- (aq) → PbI2 (s) + 2K+ (aq) + 2NO3 - (aq)
convert to total ionic equation BaCl2 (aq) + Na2SO4 (aq) → BaSO4 (s) + 2NaCl (aq)
Ba 2+ (aq) + 2Cl- (aq) + 2Na+ (aq) + So4 2- (aq) → BaSO4 (s) + 2Na+ (aq) + 2Cl- (aq)
convert to total ionic equation 2NaCl (aq) + Hg2(NO3)2 (aq) → 2NaNO3 (aq) + Hg2Cl2 (s)
2Na+ (aq) + 2Cl- (aq) + Hg2 2+ (aq) + 2NO3- (aq) → 2 Na+ (aq) + 2No3- (aq) + Hg2Cl2 (s)
net ionic equations
Molecular equations: shows all species written with full chemical formulas
Full ionic equations: shows all species as really exist in solution (i.e. strong electrolytes shown dissociated into ions)
To obtain - split up strong electrolytes
Do not split up - weak or non-electrolytes, gases, or insoluble ionic compounds
Net ionic equations: shows only the reacting species
To obtain - cancel the spectator ions form the full ionic equation
Spectator ions are ions that appear on both sides of the equation in the same form
convert to net ionic equation Pb2+ (aq) + 2NO3- (aq) + 2K+ (aq) +2I- (aq) → PbI2 (s) + 2K+ (aq) + 2NO3- (aq)
Pb2+ (aq) + 2I- (aq) → PbI2 (s)
convert to net ionic equation Ba2+ (aq) + 2Cl- (aq) + 2Na+ (aq) + SO4 2- (aq) → BaSO4 (s) + 2Na+ (aq) + 2Cl- (aq)
Ba 2+ (aq) + SO4 2- (aq) → BaSO4 (s)
convert to net ionic equation 2Na+ (aq) + 2Cl- (aq) + Hg2 2+ (aq) + 2NO3- (aq) → 2 Na+ (aq) + 2No3- (aq) + Hg2Cl2 (s)
2Cl- (aq) + Hg2 2+ (aq) → Hg2Cl2 (s)
methathesis reaction (double replacement)
AB + CD → AD + CB
Cations change partners
Charges on each ion dont change
Formulas of the products are determined by the charges of the reactants
Metathesis reactions occur only if they form a weak electrolyte or non-electrolyte as a product (otherwise, all ions are spectator ions)