GEN CHEM 1 - 2.1-2.2 and 6.1-6.4
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
State the postulates of Dalton’s Atomic Theory.
1) Elements are made of tiny particles called atoms.
2) Atoms of one element are identical while atoms of different elements are different.
3) Atoms of different elements combine together in simple proportions to create a compound.--Law of Definite Proportions or Law of Constant Composition
4) In a chemical reaction, atoms are rearranged, but not changed. --Can’t Create or Destroy Matter
2. Describe the models of atomic structure that were proposed by Thompson, by Rutherford and by Bohr and the data that supports each model.
Thomson:
Electrons are dispersed in a uniform positive charge
Used a Cathode ray tube
Rutherford:
Positive charge is not like a pudding, but concentrated in the nucleus as shown in the Gold Foil (alpha particle) experiment
Most of an atom is empty space
Bohr’s model: Structural model in which an electron moves around the necleus only in circular orbits, each with a specfic allowed radius
Electrons orbit the nucleus.
Model based on the hydrogen atom
Energy of the electrons is quantized.
3. Distinguish between matter/particles and waves, in terms of the properties of each and the dual nature of each.
Matter/Particle: Matter is anything that takes up space and mass and a particle is the smallest version of matter
Waves: Oscillation of a property over time or space; can transport energy from one point to another
Wave-particle duality: Observation that elementary particles can exhibit both wave-like and particle-like properties
The wave-particle duality of matter is a fundamental concept in quantum mechanics that suggests that all particles also have properties of waves. This means that particles like electrons and photons can exhibit behaviors typically associated with waves (like interference and diffraction) and behaviors typically associated with particles (like collisions). This duality is a cornerstone of quantum theory and is demonstrated in experiments like the double-slit experiment.
4. Define and identify wavelength(λ), frequency (f), and speed of a wave.
wavelength(λ): Distance between two consecutive peaks or troughs in a wave —> SI unit is meters
frequency (v or f): The number of wav cycles (peaks or troughs) that pass a specific point in space per unit time—> hertz
speed of a wave: the distance a wave travels in a given amount of time—> sum of λv
Wave length and frequency are inversely proportional—> as wavelength increases, frequency decrease.
5. Calculate wavelength(λ) if given frequency (f) and calculate f if given λ.
c = 2.998×108ms-1= λv
c (meter/second)= λv
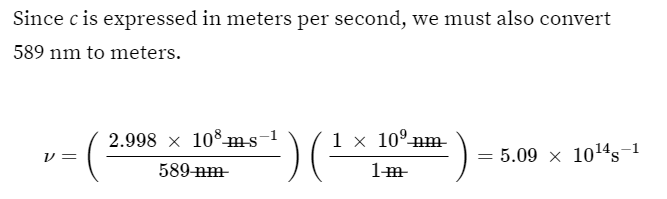
6. Be able to explain what it means to state that the energy of a wave is quantized.
Quantization: Limitation of some property to specific discrete values, not continuous—→ the system can have only certain energies and not a continuum of energies
7. State the 5 basic assumptions of Bohr’s model of the atom.
Electrons orbit the nucleus in fixed, circular paths called "stationary orbits":
Electrons can only occupy specific, discrete orbits around the nucleus and do not radiate energy while in these orbits.
Each orbit has a specific energy level:
Every orbit is associated with a fixed amount of energy, with higher energy levels further from the nucleus.
Electron energy is quantized:
Only certain energy levels are allowed, meaning electrons can only jump between specific energy states.
Electrons absorb or emit energy when changing orbits:
When an electron moves to a higher energy level, it absorbs energy (like a photon), and when it moves to a lower energy level, it emits energy (as a photon).
The angular momentum of an electron in a stationary orbit is quantized:
The angular momentum of an electron is restricted to specific values, which are multiples of Planck's constant.

8. Describe the photoelectric effect
photoelectric effect: is the emission of electrons from a material when it absorbs light of a certain frequency. Light is treated as particles called photons, and if a photon's energy exceeds the material's work function, it can eject an electron. This effect supports the particle nature of light and helped develop quantum mechanics —> E = hc/λ
Photons: smallest possible packet of electromagnetic radiation, a paticle of light
Increasing the brightness of incoming light increases the number of ejected electrons
Increasing the frequency of incoming light can increase the number of ejected electron
9. Differentiate between line spectra and continuous spectra.
Line spectra: Electromagnetic radiation emitted at discrete wavelengths by a specific atom (or atoms) in a excited state —> only shows some lines of certain colors
Continuous spectra: A unbroken series of wavelength is present —> contains all wavelengths of light in a certain range.—> shows all the color gradient
10.Know the major contributions made by the following scientists: Thompson, Rutherford, Bohr, Planck, Einstein, Rydberg, de Broglie, Heisenberg, Schrodinger, and Pauli.
Thompson: Cathode Ray Tube Experiments – beam of negative particles • Discovered Electrons – negative particles within the atom. —> Plum-pudding Model – Pudding = positive sphere – Plums = negative electrons dispersed throughout
Rutherford: Gold Foil Experiment • Discovered the nucleus – dense, positive charge in the center of the atom—> Alpha particles shot through gold foil. • Most went straight through. • A few were deflected—>• Nuclear Model – dense, positive nucleus surrounded by negative electrons
Bohr, Planck: Bright-Line Spectrum – tried to explain presence of specific colors in hydrogen’s spectrum • Energy Levels – electrons can only exist in specific energy states • Planetary Model- electrons move in circular orbits within specific energy levels Planck: found that by restricting the vibrational energies to discrete values for each frequency, he could derive an expression for blackbody radiation that correctly had the intesnity dropping rapidly for the short wavelength in the UV regoion— h is his constant which is 6.626 × 10-34Js
Einstein: used Plank constant to discover the photoelectron effect
Rydberg: Developed an empirical formula that predicted all of hydrogen’s emission lines, not just those restricted to the visible range
de Broglie: phenonmenon of wave-particle duality
Heisenberg: "uncertainty principle" in quantum mechanics, which states that it is impossible to know both the position and momentum of a particle with perfect accuracy at the same time; essentially, the act of measuring one affects the other
Schrodinger:Quantum mechanics – electrons can only exist in specified energy states • Electron cloud model – orbital: region around the nucleus where e- are likely to be found
Pauli: Pauli exclusion principle: no 2 electron in a signle atom can have the same value in all 4 quantum numbers