Biology Terms - Davenport University
1/108
Earn XP
Description and Tags
Biology midterm terms and questions
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
Discovery Science
how many well-known phenomena that are simply observations are recorded or documented
Scientific method
outlines the method and development for conducting a scientific experiment, 4 parts: observation, question, hypothesis, and experiment
1) Observation
how most scientific investigations usually begin, ex. The engine in my vehicle didn’t start
— this is a simple observation with many possible explanations
2) Question
often observation leads to this… ex. Why did the engine to my vehicle not start?
— work on one question at a time
3) Hypothesis
when scientists use the scientific method, they must develop a hypothesis, which is a “testable, tentative, and falsifiable statement that explains some observed phenomenon in nature.”
ex. The car won’t start because the battery is dead
Experiment
where you test your hypothesis you have developed
Variable
when designing an experiment, the aspect that you change is called a variable
Control
the aspect you don’t change during an experiment
Control group
receives NO variable portion of the treatment, but will have other conditions the same as the experimental group(s), reason for this is: to verify there is only ONE introduced variable that is causing chagne in the test subjects
Theory
when consistent results support a hypothesis
— a theory is a hypothesis that has been supported by experimental evidence and holds true through time
Qualitative data
these observations look at the quality of an object, such as color, shape texture
Quantitative data
these observations deal with the #/quantity of the object in question
Metric system
in biology, ALL measurements are made with this system, ALL UNITS multiples of 10
— from smaller to larger unit, move decimal to left
— from LARGER to smaller, move decimal to RIGHT
Receptor
are special nerve cells, which gather info about the outside world and the body’s internal environment
Peripheral Nervous System (PNS)
network of nerves that extends from the CNS to the REST of the body, carrying messages for sensory input and motor OUTput
Central Nervous System (CNS)
BRAIN + SPINAL CORD, acts as body’s main control center
Statistics
what scientists use to help them analyze their data and draw valid conclusions that might not be apparent based on a graph
Sample size
the number of participants, subjects, or observations in the study
Variation
the degree to which data points differ from each other and the mean/ average
Statistically significant
statistically significant results are those that are unlikely to be due to chance, the threshold for ss for an investigation, is usually a probability of 5% or less of being due to chance in order to qualify as ss
Chemoreceptors
what we owe our sense of smell and taste to
— they send info about molecules in the air to your brain, which processes the info to tell you what you’re smelling (or at least what it smells like)
Photoreceptors
what our vision depends on
— contain pigments that absorb light
Accomodation
when the lens in your eye changes SHAPE
Near point/ near point of accomodation
the limit, in terms of how close they can bring an object and still see it clearly
Standard Deviation
indicates how “spread out” the values are in a set of data, specifically, standard deviation measures , on average, how much each observation differs from the mean
— if the values are clustered around the mean, then the SD is low
Mechanoreceptors
special receptor cells that are in your SKIN that collect different types of info, they’re tuned in to touch and pressure, your sense of touch is related to both the # and depth of receptors in your skin
International System of units (SI)
What the modern metric system is, so…
meter (m) for length
liter (l) for volume
gram (g) for mass
Scientific Notation
a number is expressed in scientific notation when it is written as a product of a decimal number between 1 and 9 and the number 10 raised to the proper power
Significant figures
ALL certain digits, plus the next estimated digit
Accuracy vs. Precision
Accuracy— the degree to which an observed value corresponds to the true value
Precision— the degree to which measurements are reproducible when repeated
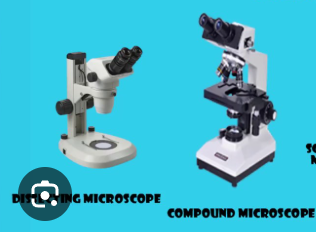
Compound Microscope vs. Dissecting Microscope
Compound— magnification of up to 400x to 1000x or more, more detailed observations, thin, transparent, translucent samples that light can pass through ex. bacteria, individual cells, or plant tissues
Dissecting— magnification of up to 70x or 100x, ideal for viewing surface details of larger objects, opaque, 3D specimens that you can see with the naked eye, ex. insects, flowers, animals, produces a stereoscopic 3D image with a sense of depth
Resolving power
A second lens within the objective is responsible for limiting its resolving power, the ability to reveal detail, to distinguish 2 closely spaced objects as being 2 rather than 1
— the smaller the distance btwn 2 objects that can be distinguished from one another, the better the resolving power of the instrument
Angular aperture (θ) vs. Numerical aperture (NA)
the angle between the optical axis and the most oblique light rays that the objective lens can capture from the specimen
— when angle, θ is too small, the resolution is poor
— higher the #, better the microscope
— sharper the cone, better the microscope
Angular aperture is the total angle of the cone of light a lens can collect
Numerical aperture (NA) is a dimensionless measure that includes both the angular aperture and the refractive index of the medium between the lens and the specimen
— the expression R= λ/ 2 NA, numerical aperture is a pure number, it is unitless, NA is engraved on the side of ALL objectives next to the number indicating magnification
— higher the NA value, the smaller the R (resolution) will be, the smaller the R, the better the resolution of the objective (and more expensive)
Refractive Index (n)
the medium through which the light must travel will affect the cone of light and thus resolution, air has a refractive index of n = 1. Oil has a greater refractive index than air (n=1.5) and is often used to increase resolution of the microscope at higher powers by increasing the ange (θ) of the cone of light that passes into the objective
Wavelength of light (λ)
the shorter the wavelength of light, the greater the resolution of the objective, the value of (λ) can be changed by using colored filters
Ocular lens/ eyepiece
the lens you look through, usually magnify objects to 10 times their size (10x), some cases the body tube can be rotated for others to see more easily
Stage
holds the slide to be viewed, can be moved vertically by turning the coarse adjustment knob and fine adjustment knob
Stage clips
hold the slide so that it can be moved by hand, adjustment knobs are used to move the slide in the horizontal plane, so side to side
Base and Arm
important support parts of the microscope that allow for easy carrying
Coarse adjustment knob
used for initial focusing of specimens at low power
Fine adjustment knob
makes very slight changes, allowing precision focusing at higher power
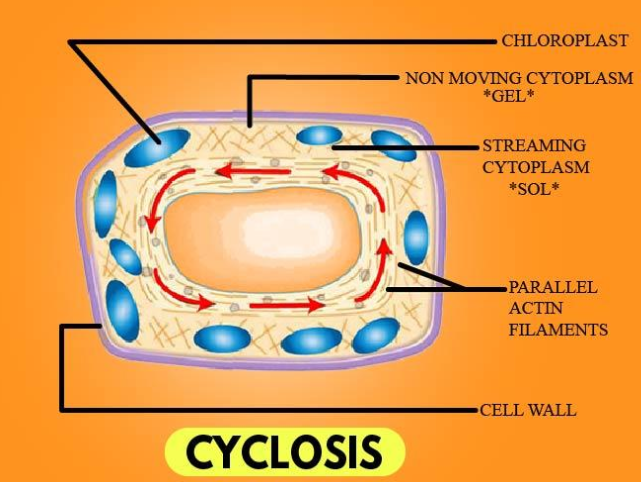
Cyclosis (cytoplasmic streaming)
the movement you see in the chloroplasts of Elodea leafs, the chloroplasts are responsible for photosynthesis in plant cells, as the cytoplasm moves around the large central vacuole, it carries with it dissoved substances as well as suspended organelles
Plasmolysis
when cytoplasm shrinks from the cell walls
Transmitted light vs. Reflected light
transmitted light passes through a specimen, used for transparent or translucent specimens
— light can be transmitted through a specimen on the microscope stage by proper adjustment of the mirror below the stage
reflected light bounces off it, used for opaque specimens like rocks, coins
— light can be reflected from the surface of a specimen by positioning the light source above the specimen so that it shines downward onto the microscope stage. If your illuminator is built into the microscope, light will be adjusted by 2 switches: the transmitted light switch and the reflected light switch
Organic compounds
carbs, lipids, proteins, nucleic acids, they are composed of the elements carbon and hydrogen (hydrocarbons)
Monosaccharides + Polysaccharides
building blocks of carbs, ex. glucose, fructose, lactose
— building block of larger carbs called polysaccharides, ex. starch, glycogen, cellulose-forms cell wall of plants
Starch is how plants store glucose
while animals and humans store glucose in the form of glycogen
Amino Acids, Peptides, & Peptide bonds
— Proteins that are comprised of small molecules are amino acids
— A chain of amino acids are called peptides
— Amino acids linked together to form chains with a special type of covalent bond known as peptide bond
Lipids
includes molecules that are identified as greasy, fatty, oily, and are generally insoluble in water
ex. fats/oils, phospholipids, sterols
fats and oils are comprised of compounds known as fatty acids which are attached to a glycerol molecule, if 3 fatty acids are attached to the glycerol, known as a triglyceride
Positive and Negative controls
Positive (+) control - presence of a positive biological chemical reaction to a known organic molecule
Negative (-) control - the complete absence of any organic molecule (distilled water)
Biuret reaction
test used for specific detection of proteins, reaction of a blue solution containing copper sulfate (CuSO4)
Iodine Potassium Iodid (IKI) reaction
detection of starch presence is achieved by iodine present in a dark amber solution of IKI. The iodine molecules slide into and interact with the coils of starch and change the color into blue-black thus indicating the presence of that organic molecule
Benedict’s Test
detection of monosaccharides/disaccharides by another copper-based test, this method relies on the ability of the reactive mono- and disaccharides to give up electrons and be oxidized in the presence of Cu+ ions, when Cu++ is reduced to Cu+, the reaction will change color from blue to brick red
Hydrolysis
the chemical breakdown of a compound due to a reaction with water
Amylase (enzyme)
converts starch and glycogen into simple sugars, amylase catalyzes this process and is found in saliva and the digestive system
Denaturation
a process where a complex molecule, most often a protein or DNA, loses its native three-dimensional structure and function due to external factors like heat, extreme pH, or chemicals
This unfolding is often irreversible and results in the loss of the molecule's specific biological properties, such as an enzyme's ability to bind a substrate.
PH
pH is a scale from 0 to 14 that measures how acidic or alkaline (basic) a solution is. A pH of 7 is neutral, while a pH less than 7 is acidic and a pH greater than 7 is alkaline. It is calculated as the negative logarithm of the hydrogen ion concentration pH=−log[H+]p cap H equals negative log open bracket cap H raised to the positive power close bracket
𝑝𝐻=−log[𝐻+] and is a crucial factor in many chemical and biological processes.
Ionic strength
a measure of the total concentration and charge of all ions in a solution, reflecting how these ions interact with each other
Charge shielding: A higher ionic strength shields the charges on molecules, which can affect electrostatic interactions, and influence protein folding, aggregation, and activity.
Reaction rates: It influences the rates of reactions, especially those involving charged particles, by affecting how ions interact.
Buffer performance: Ionic strength impacts a buffer's ability to maintain a stable pH by affecting the activity coefficients of its components.
I=1/2∑(CiZi²)
ex. For a 0.01 M NaCl solution:
concentrations are 0.01 M for Na+ & 0.01 M for Cl-
charges are +1 for Na+ and -1 for Cl-
I = ½ x (0.01 × 1² + 0.01 x (-1)²) = 0.01 M
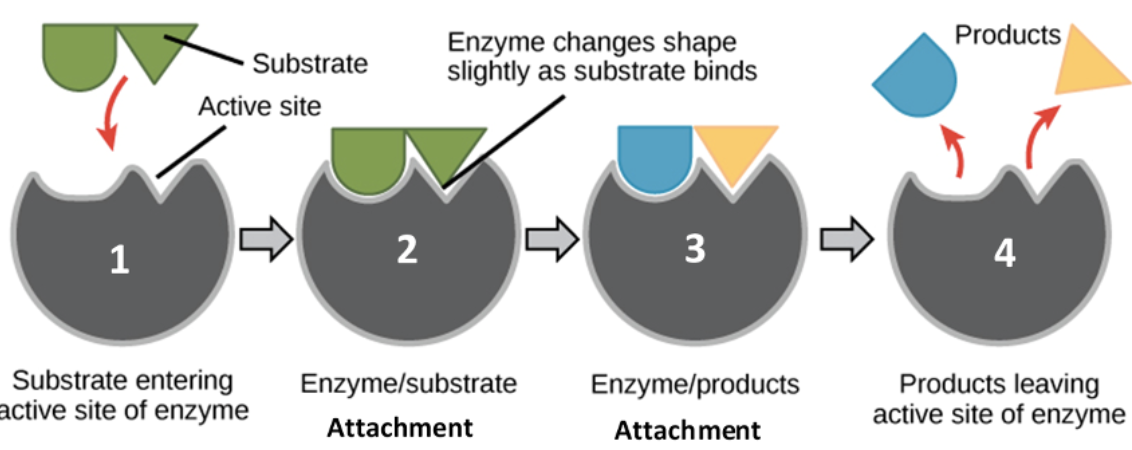
Active Site
the specific region on an enzyme where a substrate binds and the chemical reaction takes place
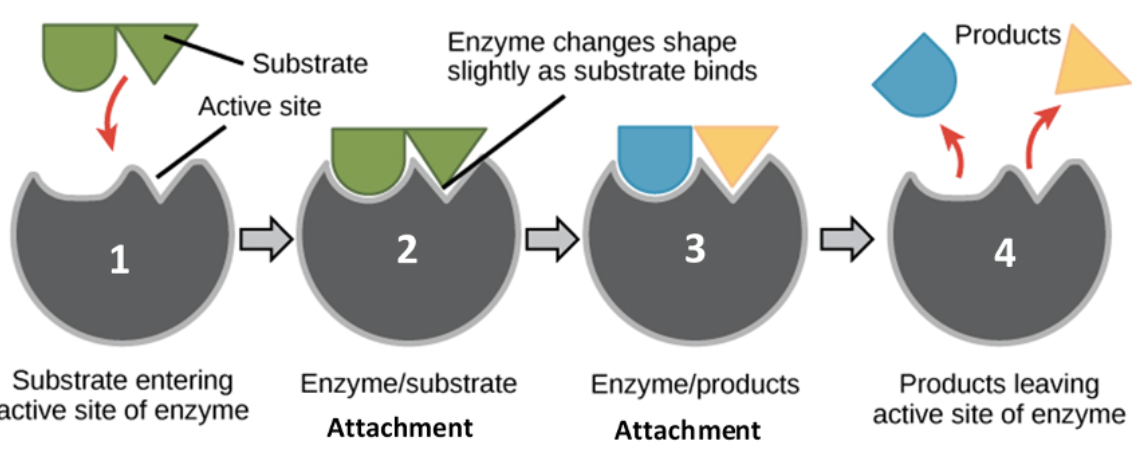
Substrate
a molecule upon which an enzyme acts, or the surface on which an organism lives
Unified Cell Theory
All living organisms are composed of one or more cells: This means that both single-celled and multicellular organisms are made of cells.
The cell is the basic unit of life: The cell is the smallest and most fundamental unit of structure and organization in all living things.
All new cells arise from pre-existing cells: Cells do not come into existence spontaneously; they are created from the division of other cells.
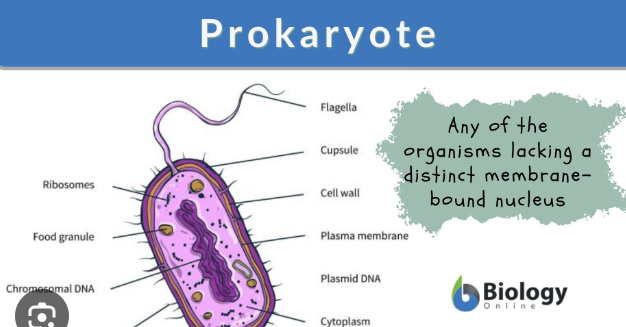
Prokaryote “pro think no membrane-bound”
a single-celled organism that lacks a membrane-bound nucleus and other organelles. Instead of a nucleus, its genetic material is in a single, circular chromosome located in the cytoplasm's nucleoid region. Examples include bacteria and archaea.
Eukaryote - “eu think do”
an organism whose cells contain a membrane-bound nucleus and other membrane-bound organelles, such as mitochondria
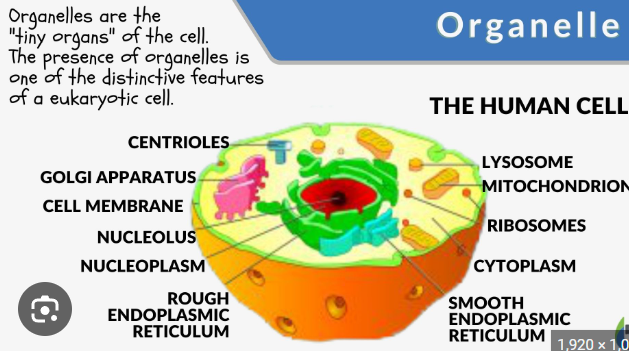
Oraganelle
a specialized subunit within a cell that has a specific function, much like an organ in a body
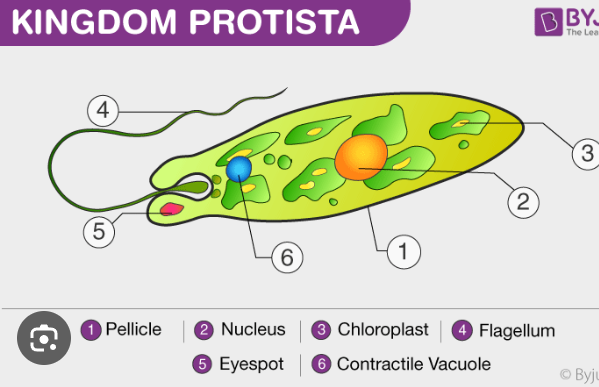
Protist
a diverse group of eukaryotic organisms that are not animals, plants, or fungi. They are often single-celled, but some can be multicellular or form simple colonies, and they have a wide range of nutritional and reproductive methods. Examples include algae, amoebas, and slime molds.
What are the dress requirements for lab safety?
Closed-toed shoes, hair tied back, no loose clothing that could get caught, pants
What is the purpose of safety showers? Eyewash stations? Fume hoods?
Safety showers– these provide 1st aid, wash for 15 min
Eye wash stations– provide 1st aid, rinse for 15 min
Fume hoods– designed to prevent dangerous fumes, gases, and vapors from entering the lab
What is the difference between chemicals, solutions, and reagents?
Chemicals– are single chemical compounds of known purity and composition
Solutions– consist of a chemical (solute) dissolved in another (solvent), a solution label will contain the name of the chemical, its concentration, and the solvent
Reagents– are ALL prepared chemicals and solutions used to detect, analyze, and make other substances, they can be as simple as a weak acid solution (e.g., 0.10 M HCL) or complex mixtures of a number of chemicals, such as Yamada’s Universal indicator, a solution of 5 different acid-base indicators
What are the 3 “NEVERS” to consider when handling reagent bottles?
NEVER set the cap to the reagent bottle down. Hold it between the 2nd and 3rd fingers of 1 hand.
NEVER insert objects (including pipettes) into reagent bottles. Instead, transfer some of the reagent to a clean beaker and pipette from this container. EXCEPTION: in those cases where there is a designated transfer pipette associated with the reagent bottle, this pipette may be inserted into the bottle
NEVER return unused reagent to the reagent bottle. DISPOSE of ALL unused reagent as chemical waste
Where would you dispose of solid chemical wastes? Heavy metal water solutions? Glass waste?
Solid chemical waste– ALL solid chemicals remaining at the end of the experiment MUST be put in the designated container, used transfer pipettes are considered to be solid chemical waste and should be disposed of as such
Heavy metal water solutions– a separate container is provided for heavy metal waste, NEVER pour these down the drain or liquid organic waste/ acid and basic water solutions
Glass waste– ALL glass waste MUST be put in the designated glass waste container. This includes glass with chemicals on it, such as capillary tubes, glass TLC plates, broken flasks, etc.
Be able to recognize the difference between an example of an observation, a question, a hypothesis, and a prediction.
Observation– 1st step in scientific investigations
Question– often an observation leads to this
Hypothesis– a tentative, testable, and falsifiable statement that explains some observed phenomenon in nature
Prediction– what we expect from experimental results if the hypothesis is correct
Be familiar with the difference between a variable and a control when given example experiments.
Variable– what we change in the experiment
Control– what stays the same in an experiment
Control groups– receive NO variable portion of the treatment, but will have all other conditions the same as the experimental group(s)
Recognize different quantitative and qualitative observations
Quantitative– #/ deal with the quantity of some aspect of the object in question
Qualitative– look at the qualities of an object, color, shape, and texture
How do you convert a milligram to a gram to a kilogram? Be able to convert between different metric size units
From mg to g x 1,000 From g to kg divide by 1,000
Understand why specialized senses may prove advantageous in the process of natural selection
They improve an organism’s ability to survive and reproduce in a specific environment to help find food, shelter
Be familiar with the process of communication from receptors to the central nervous system
nerves in the PNS pass info up from the receptors (nerve cells) along to the brain + spinal cord which makes up the CNS
Why are statistics necessary in the process of formulating results and drawing experimental conclusions? How do sample size and variation in data points play a role in this analysis process?
Statistics help scientists analyze their data and draw valid conclusions that might not be apparent based on a graph. Ex. A larger sample size generally increases the precision and reliability of the results, while greater variation requires a larger sample to achieve the same level of confidence
What is the role of chemoreceptors in the sense of smell?
Owe sense of smell and taste to these, they send info about molecules in the air to your brain
Be familiar with the use of a t-test and how a p-value can be used to conclude the data of two means (averages)
A t-test is used to compare the means of 2 groups to determine if they are statistically significant.
A p-value indicates the probability of observing the difference between 2 sample means if the true population means were equal (the null hypothesis)
Ex. If p value is below a pre-determined significance level (alpha), the you reject the null hypothesis and conclude that a statistically significant difference exists, otherwise you fail to reject it
Statistical significance– the probability that a result is NOT due to random chance, meaning it likely represents a genuine effect
How does the eye adjust focus as an object moves toward or away from the eye? What is the name of this phenomenon?
You can see clearly thanks to the lens of each eye, which can change shape. When the lens changes shape, this is accommodation, the near point of accommodation is the limit to how close people can bring an object close and still see it clearly
Be familiar with how to calculate a mean in a data set, and what standard deviation tells us about that data set.
mean= add up #’s, divide by how many #’s there are
Standard deviation– tells us how “spread out” the values are in a set of data, specifically, standard deviation measures, on average, how much each observation differs from the mean, if values are clustered around the mean, the standard deviation will be low
What is the role of mechanoreceptors in the skin? Be familiar with how to measure a two-point threshold.
Mechanoreceptors are tuned in to touch and pressure. These are special receptor cells in your skin that collect different types of information.
called a Caliper
This is how to measure a 2-point threshold

Understand the reasoning for why some areas of the body are more sensitive to touch than others.
Some are more sensitive than others due to a higher density of nerve endings/ # and depth of receptors in skin and a larger representation on the brain
Be able to calculate the range in a data set
Biggest # - smallest # = range
When given a formula, be able to convert from Celsius to Fahrenheit, and back.
Celsius to Fahrenheit F= 9/5 (C) + 32
Fahrenheit to Celsius C= 5/9 (F) - 32
What is the name of the region of liquid where you read a volume in a graduated cylinder?
meniscus
When are some measurement tools (beaker vs graduated cylinder vs volumetric flask) more appropriate than others?
Volumetric flask when measuring single exact amts of volumes, more accurate than graduated equipment
Use beaker for general, non-precise tasks like mixing, heating, or cooling liquids
Use graduated cylinder for more precise and accurate volume measurements
Why is it necessary to “tare” a balance when you use it?
Ensures accurate measurements by setting the scale to zero, which ignores the weight of the container
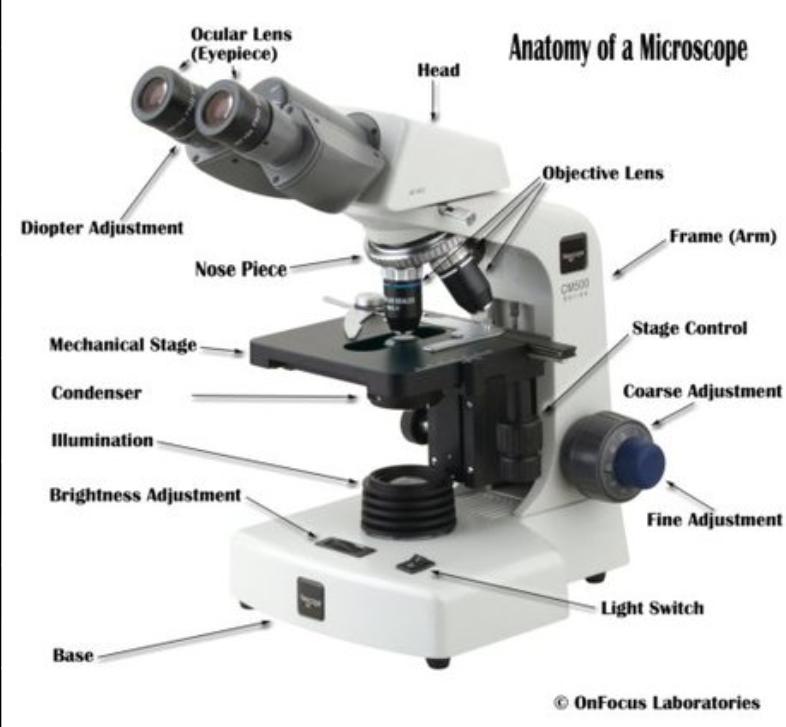
Be familiar with the parts of the microscope and the purpose of each
Ocular objectives– magnify image, usually 10x to 15x
Head– holds ocular lenses
Arm– holds head and stage
Mechanical stage knobs– move slide on stage (both horizontal and vertical knobs)
Fine adjustment knob– sharpens image
Revolving nosepiece– rotates objectives into viewing position
Slide holder– fixed and movable parts
Stage– holds slide
Condenser– focuses light on specimen
Iris diaphragm– controls amount of light entering specimen
Light– illuminates specimen
What factors influence the resolving power of a microscope? Know what each entails, not just the key term involved
3 factors:
Angular Aperture (theta), sharper the cone, the better the microscope, the higher the #, better the resolution, def: the angle that a lens/ optical system subtends from a focal point, representing the maximum angle of light rays it can capture
Refractive index (n)- a measure of how much light bends, or refracts, when passing from a vacuum into a material, it is the ratio of the speed of light in a vaccum to the speed of light in the material, meaning a higher refractive index indicates that light travels more slowly through the material, the medium through which light must travel will affect the shape of the cone of light and thus resolution. Air has a refractive index of n=1. Oil has a greater refractive index than air at n=1.5, and is often used to increase the resolution of the microscope at higher powers by increasing the angle (theta) of the cone of light that passes into the objective
Wavelength of light (𝜆) – the shorter the wavelength of light, the greater the resolution of the objective. The value of 𝜆 can be changed by using colored filters, the longer the wavelength, the less powerful the resolution
Be able to calculate the total magnification of a microscope.
Multiply the magnification of the objective lens by the magnification of the ocular lens
Objective lens usually 4x, 10x, 40x, ocular is typically 10x, so multiply them together
When choosing an objective lens, which numerical aperture is more desired?
A higher NA is generally more desired because it provides better resolution, meaning the ability to distinguish between closely spaced objects, results in a brighter, clearer image, a higher NA comes with a shorter depth of field, which can be a trade off depending on the application
When you added salt to the elodea leaf, what happened to the cells?
Water went from lower conc. (inside the cell) to higher conc. (outside the cell), plasmolysis occurred so which caused the cell membrane to shrink away from the rigid cell wall, as a result, the cytoplasm and chloroplasts clustered in the center of the cell
How do you prepare a wet mount slide?
Get a clean slide and cover slip
Add a drop of liquid, use pipette or eye dropper to place a single drop of water or other liquid in the center of the slide
Place the specimen, using forceps or another tool, place a very thin sample of specimen into the drop of liquid on the slide, if already in liquid form, just place a drop in the middle of the slide
Hold coverslip at 45 degree angle, with one edge touching the glass slide near the liquid droplet
Use edge of paper towel to blot out excess liquid, the coverslip should not be floating but held in place by the surface tension of the liquid
Which is better when using a dissecting microscope to observe a large specimen? A transmitted light or a reflected light?
Reflected light, this shines onto the specimen from above, allowing you to view the surface details of thick, opaque objects like insects, rocks, or plants
What are organic molecules? What sets them apart from other molecules?
Are chemical compounds that contain carbon, typically bonded with hydrogen, oxygen, nitrogen, and other elements, they are distinguished from inorganic molecules bc their structure is built around chains or rings of carbon atoms, they usually contain carbon-hydrogen bonds, a defining feature absent in inorganic compounds like CO2
Know the 4 classifications of organic molecules and be able to recognize each by its chemical structure
Carbohydrates, lipids, proteins, nucleic acids
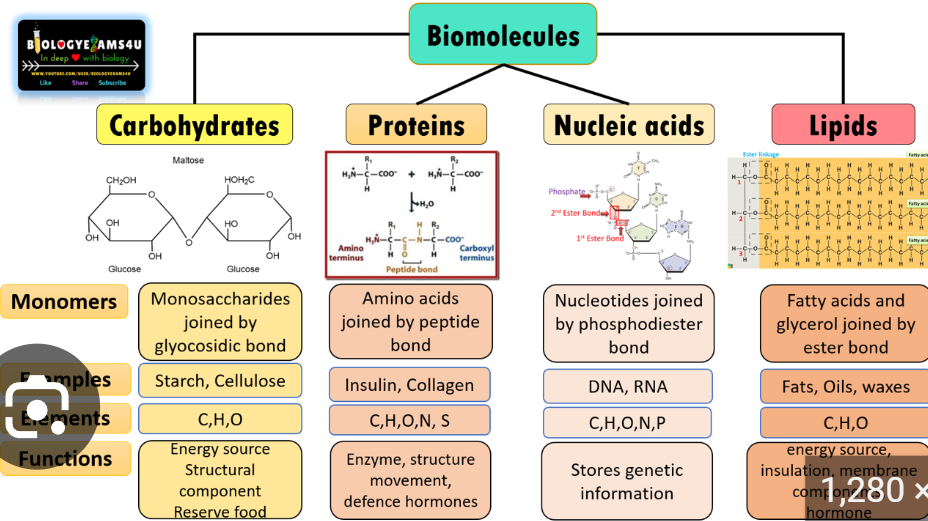
What is the difference between a monosaccharide and a polysaccharide? What are examples of each?
Monosaccharides are building blocks of carbs, ex. Glucose, fructose, lactose, also building blocks of larger carbs called Polysaccharides
Ex. starch and glyocogen
Plants store glucose as Starch
Animals/humans store glucose as glycogen
Be familiar with the IKI test and how starch is detected.
IKI test detects the presence of starch through a color change, when iodine which is yellowish-brown, is added to a sample containing starch, it turns a deep blue or blue-black color
How do enzymes work in catalyzing a chemical reaction?
Enzymes work by lowering the activation energy of a chemical rxn, a process that significantly speeds up the rate of reaction
Be familiar with the relationship between an enzyme, a substrate, and a product.
An enzyme acts as a catalyst, binding to a specific substrate at its active site to form an enzyme-substrate complex. This binding facilitates a chemical reaction that converts the substrate into a different molecule, the product, which is then released from the enzyme, allowing the enzyme to bind to another substrate