Calculations Exam Review
1/109
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
110 Terms
How many grams are there in fifty 0.4 mg atropine sulfate tablets? Round your answer to 2 decimal places
0.02 g
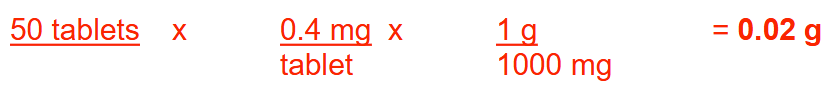
How many milligrams of nitroglycerin are there in 60 tablets if each tablet contains 1/100 grain of nitroglycerin? Round your answer to the nearest whole number.
39 mg
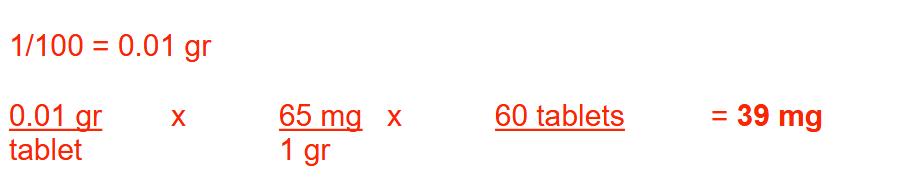
A pharmacist, on separate occasions, dispenses 220 mg and 450 mcg of a particular drug. How many grams of the drug remain if the container originally contained 1.5 g of the drug? Round your answer to 2 decimal places
1.28 g
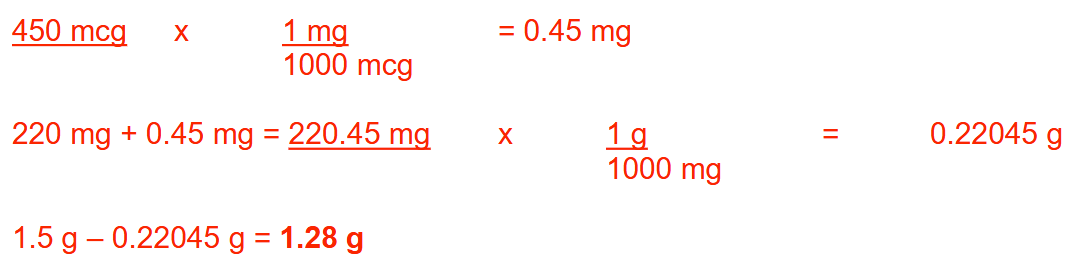
An inhalation aerosol contains 0.03 g of albuterol sulfate per canister and is labeled to deliver 200 full inhalations. If each inhalation contains 108 mcg of albuterol sulfate, how many milligrams of drug would remain in the canister once all 200 inhalations are administered? Round your answer to 1 decimal place.
8.4 mg

A liquid contains 0.25 mg of a substance per milliliter. How many grams of the substance will be present in 3.5 L of this liquid? Round your answer to 2 decimal places.
0.88 g

A soft gelatin capsule contains 0.22 mL of eucalyptus oil. How many liters of this oil will be required to make 4,600 capsules? Round your answer to 2 decimal places.
1.01 L

You currently have 15.8 g of an antibiotic powder available. How many 250-mg capsules of this antibiotic can you make from this quantity of powder?
63 capsules

A pharmacist adds sufficient water to a vial containing 2 million units of penicillin G to make a total volume of 5 mL of solution. How many milliliters of penicillin G solution should be administered to a child who is to receive a dose of 300,000 units? Round your answer to 2 decimal places.
0.75 ml
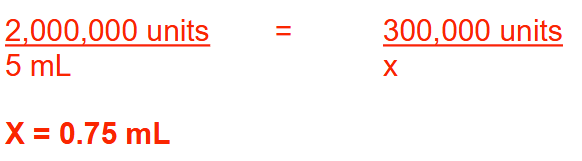
A child receives 1/10 fl oz, 1/8 fl oz, 3/8 fl oz, 0.2 fl oz, and 0.15 fl oz of a cough syrup. How many total milliliters of cough syrup did this child receive? Round your answer to 1 decimal place.
28.5 ml

Five hundred penicillin VK tablets cost $43.09. What is the cost of 48 penicillin VK tablets? Round your answer to 2 decimal places
$4.14
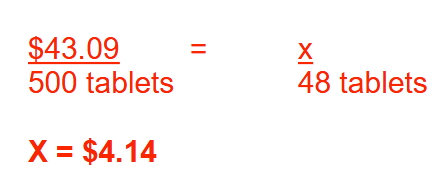
How many grams of hydrocortisone powder are needed to prepare 100 g of the following ointment?
Round your answer to 2 decimal places.
Rx:
Mineral oil 6 parts
Nystatin powder 1 part
Hydrocortisone powder 2 parts
Zinc oxide ointment 200 parts
0.96 g
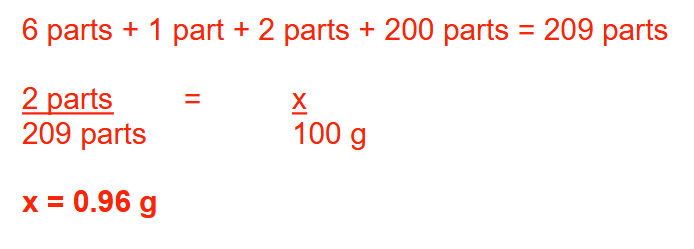
How many grams of lanolin are needed to prepare 1 lb of the following cream? Round your answer to
1 decimal place.
Rx:
White wax 12.5 g
Mineral oil 60 g
Lanolin 2.5 g
Sodium borate 1 g
Rose water 28 g
10.9 g

The following formula is used to make a 4-g packet of an effervescent powder:
Rx:
Sodium bromide 6 parts
Sodium bicarbonate 18 parts
Tartaric acid 10 parts
Citric acid 5 parts
How many ounces of sodium bromide will you need to prepare 200 of these packets? Round your answer to 1 decimal place.
4.1 oz

How many fluid ounces of propylene glycol are needed to make two dozen 15-mL bottles of the following nasal spray? Round your answer to 1 decimal place.
Rx:
Testosterone 1 g
Alcohol 10 mL
Propylene glycol 20 mL
Benzalkonium chloride 15 mg
Purified water qs ad 100 mL
2.4 fl oz

A nasal spray is supplied as a 10-mL bottle that delivers 25 sprays per milliliter, with each spray containing 2 mg of drug. How many micrograms of drug are contained in each bottle? Round your answer to the nearest whole number.
500000 mcg
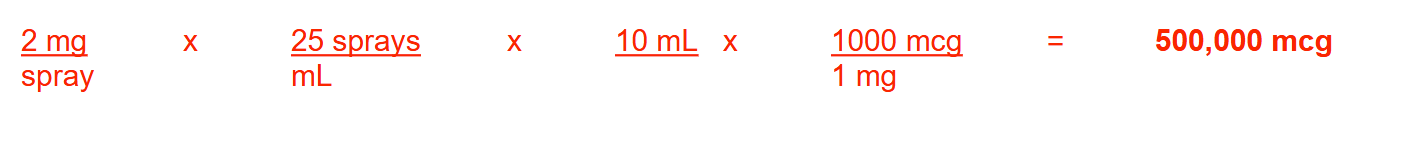
If 1 L of alcohol weighs 1010 g, what is its density? Round your answer to 2 decimal places
1.01g/ml

If 469 mL of liquid weighs 697 g, what is its specific gravity? Round your answer to 2 decimal places.
1.49
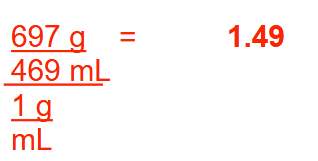
What is the weight (in grams) of 2970 mL of a liquid that has a specific gravity of 0.967? Round your answer to 2 decimal places.
2871.99 g
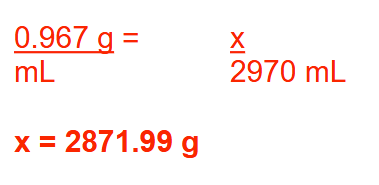
What is the volume (in liters) of 685 g of a substance that has a specific gravity of 1.68? Round your answer to 2 decimal places
0.41 L
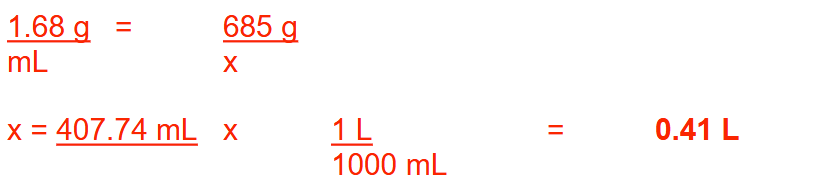
What is the % (v/v) concentration of a solution if 470 g of an acid (specific gravity = 0.675) is mixed with enough water to make 2 gallons of solution? Round your answer to 1 decimal place
9.2%
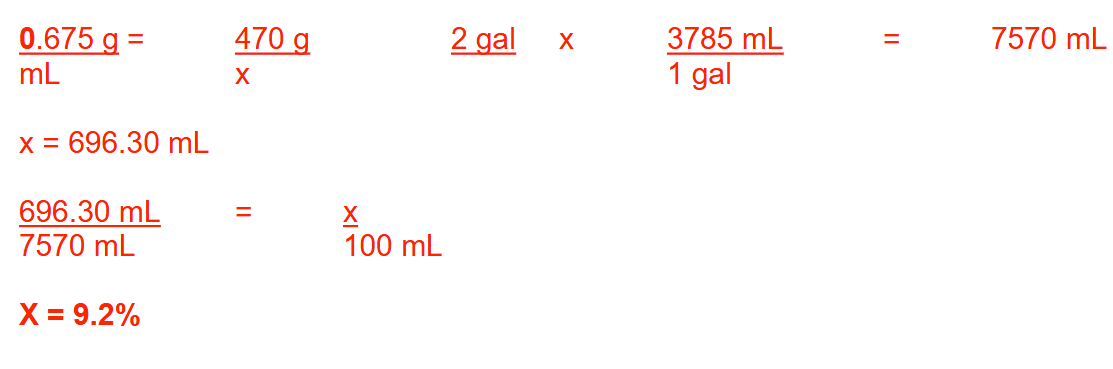
What is the volume (in quarts) of 25 lbs of peppermint oil having a specific gravity of 0.908? Round your answer to 1 decimal place.
13.2 quarts
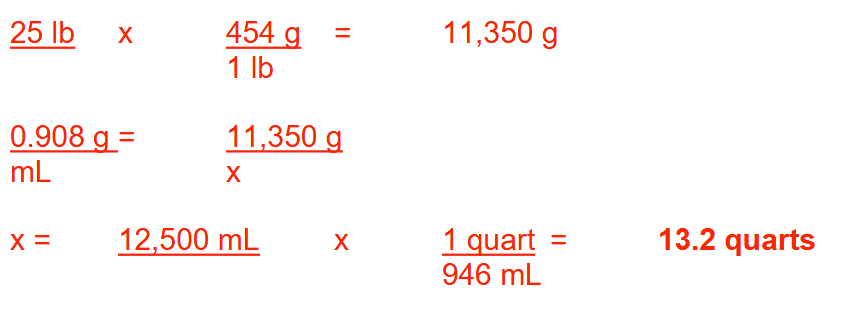
How many milligrams of hydrocortisone powder are needed to prepare 2 pounds of a 2% (w/w)
hydrocortisone ointment? Round your answer to a whole number.
18160 mg
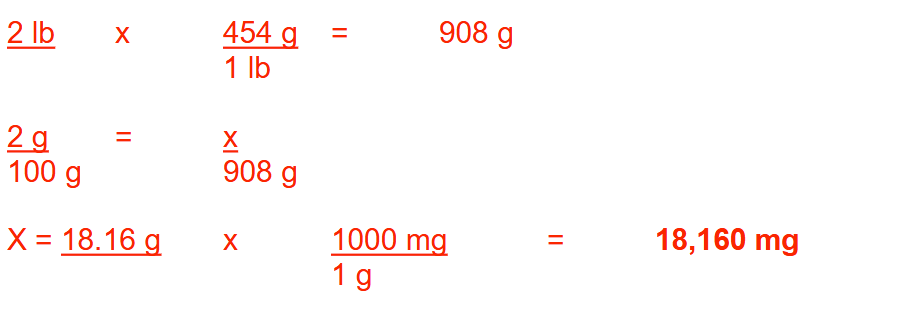
A sodium fluoride solution is prepared by dissolving 50 mg of sodium fluoride in enough water to make 100 L of solution. Express the concentration of the solution as a ratio strength (w/v).
1:2,000,000

What is the ratio strength (w/v) of a 0.25% (w/v) solution?
1:400
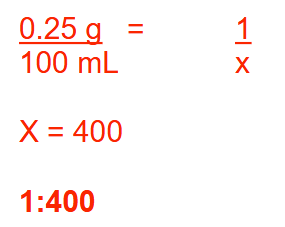
How many milligrams of sodium fluoride are needed to prepare 1 gallon of a 2 ppm (w/v) solution? Round your answer to 2 decimal places
7.57mg

A solution is prepared by adding 50 g of calcium chloride (MW = 111) to enough water to make 650 mL of solution. Express the concentration of the solution as % (w/v). Round your answer to 1 decimal place.
7.7%
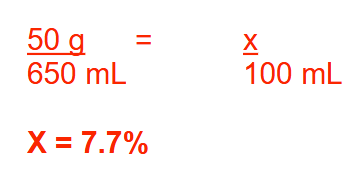
Each gallon of a dilute alcohol solution contains 500 mL of absolute ethanol. What is the % (v/v) concentration of ethanol in the solution? Round your answer to 1 decimal place
13.2%
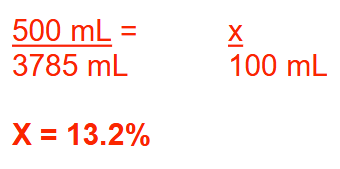
How many milliliters of a 1:100,000 (w/v) sodium fluoride solution are needed to provide a 50-mcg dose? Round your answer to a whole number
5 ml

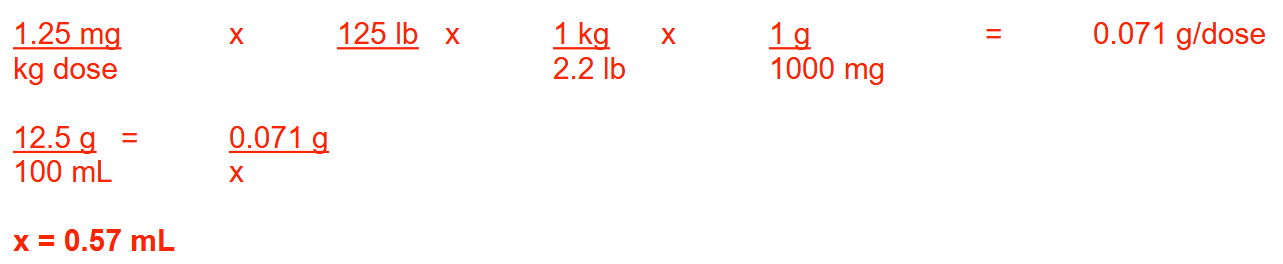
The usual daily dose of an intravenous antibiotic is 2.5 mg/kg/day divided into 2 equal doses. How many milliliters of a 12.5% (w/v) solution of this antibiotic should be added to a 100 mL bag of normal saline and administered to a patient weighing 125 lb to provide one dose? Round your answer to 2 decimal places.
0.57 ml
A pharmacist dissolves the contents of 8 capsules, each containing 300 mg of clindamycin phosphate, into a sufficient amount of an astringent liquid base to prepare 60 mL of topical solution. What is the % w/v concentration of clindamycin phosphate in this prescription? Round your answer to a whole number.
4%
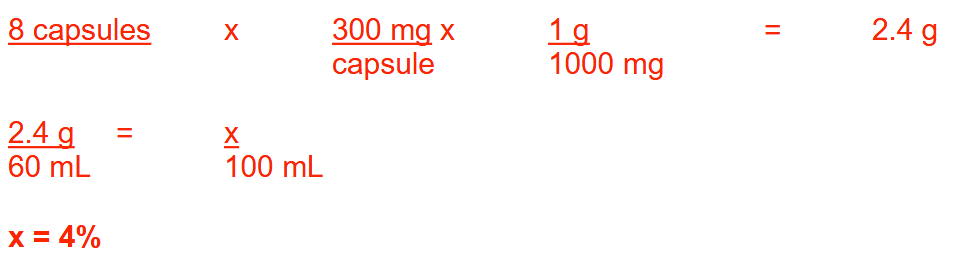
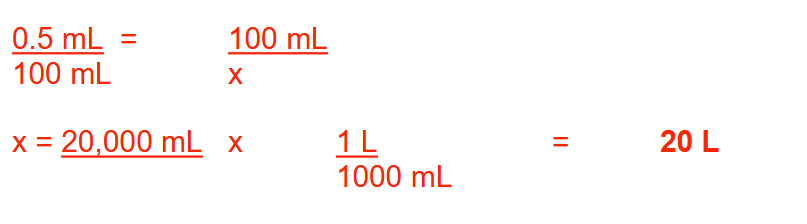
How many liters of a mouthwash can be prepared from 100 mL of cinnamon flavor if its concentration is to be 0.5% (v/v)? Round your answer to a whole number.
20 L
If a city water supply has a limit of 250 ppm (w/v) of nitrate ion, what is the maximum amount of nitrate ion, in grams, that may be present in a 10,000-gallon reservoir? Round your answer to 1 decimal place.
9462.5 g

You receive the following prescription to be compounded:
Rx:
Hydrocortisone
Hexachlorophene aa 0.25% (w/w)
Coal tar solution (1:25 w/v) 10 mL
Hydrophilic ointment qs ad 120 g
How many tablets, each containing 20 mg of hydrocortisone, should be used in preparing this prescription?
15 tablets

How many fluid ounces of solution can be prepared from 42.3 mg of an antioxidant if the final concentration of the antioxidant is supposed to be 12 ppm (w/v)? Round your answer to 1 decimal place
117.5 fl oz

Simethicone drops contain 2 g of drug in a 30-mL bottle. How many milliliters of the drug would provide a 40-mg dose? Round your answer to 1 decimal place
0.6 ml
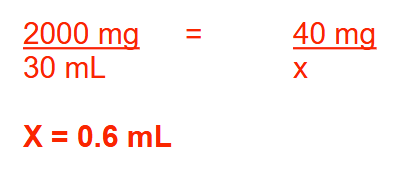
An ophthalmic solution contains 0.15% brimonidine tartrate in 10-mL containers. The recommended dose is 1 drop in the affected eye(s) 3 times daily. If a patient with glaucoma instills the recommended dose into each eye, and the dropper used delivers 20 drops/mL, how many milligrams of brimonidine tartrate does the patient receive each day? Round your answer to 2 decimal places
0.45 mg

A patient presents to the pharmacy with the following prescription:
Amoxicillin oral suspension 125 mg/teaspoon
Sig: 250 mg PO QID x 10 days
If a bottle containing 1 pint of reconstituted amoxicillin suspension was dispensed to this patient, how many fluid ounces would be left in the original container once the treatment course is completed?
Round your answer to 1 decimal place
2.4 fl oz
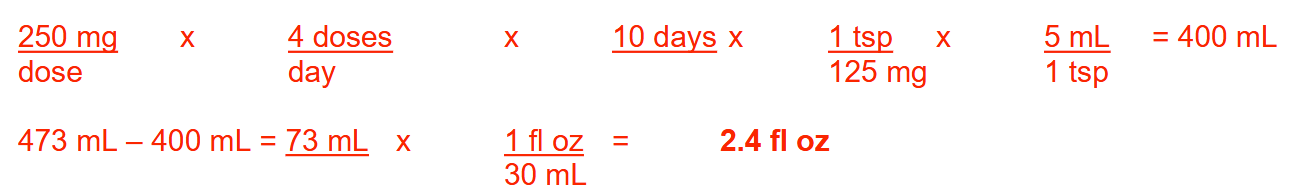
How many 145-mg fenofibrate tablets can be made from 12 g of the drug?
82 tablets

The dose of a drug is 10 mg/kg of body weight. How many micrograms should be given to a child weighing 60 lb? Round your answer to 2 decimal places
272,727.27 mcg

The adult dose of a liquid medication is 0.2 mL/kg of body weight to be administered as a single dose.
How many teaspoonfuls should be administered to a person weighing 225 lb? Round your answer to a whole number
4 tsp
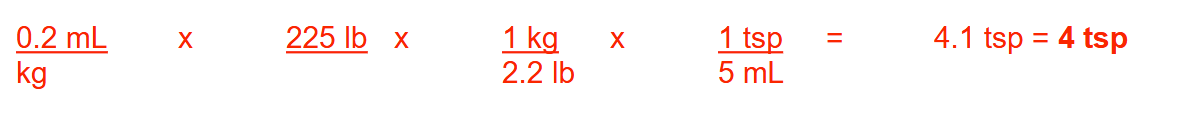
How many tablets, each containing 150 mg of Drug X, are needed to provide 20 mg/kg/day for 10 days for a person weighing 134 lb?
82 tablets

If a 4-year-old child weighing 40 lb accidentally ingested twelve 81-mg aspirin tablets, how much aspirin did the child ingest on a milligram per kilogram basis? Round your answer to 1 decimal place.
53.5 mg/kg

The dose of mitomycin injection for gastric cancer is 20 mg/m 2 once every 6-8 weeks. Determine the dose in milligrams that a patient who weighs 175 lb and is 5’7” tall should receive each time the drug is administered. Round your answer to 1 decimal place.
38.7 mg
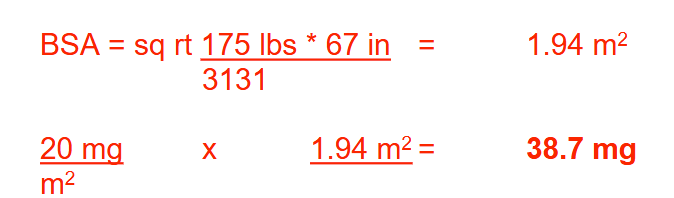
You are the oncology pharmacist in the infusion center at a large academic medical center. A 72 year-old man (5’9”, 189 lb) presents today for his first chemotherapy treatment after being diagnosed with esophageal cancer. The physician has prescribed IV cisplatin at a dose of 200 mg for this cycle of this patient’s chemotherapy. Based on a drug information reference, the recommended dose of cisplatin for esophageal cancer is 60 mg/m 2 for each cycle of chemotherapy. Is the prescribed dose of cisplatin appropriate for this patient?
122.45 mg, prescribed dose too high

How many milligrams of penicillin VK should a 41-pound child receive with each dose if the dosing regimen is 75 mg/kg/day in divided doses every 6 hours? Round your answer to 1 decimal place
349.4 mg

ow many milligrams of daunorubicin would a patient (5’9”, 56 kg) receive over 3 days of therapy if the regimen called for 45 mg/m2/day x 3 days? Round your answer to 1 decimal place.
222.9 mg

A prescription is written for a child who weighs 52 lb to receive acetaminophen 10 mg/kg PO Q4h. How many grams of acetaminophen will the child receive each day? Round your answer to 2 decimal places
1.42g/day

You receive a prescription for fluticasone HFA 440 mcg BID with instructions to dispense a 90-day supply. Fluticasone HFA is available commercially as 220 mcg/metered dose with 120 metered doses per inhaler. How many inhalers should be dispensed for a 90-day supply?
3 inhalers

An IV solution contains 500 mcg/mL of a drug. How many milligrams of the drug would this patient receive with infusion of 1.5 liters of this drug? Round your answer to a whole number.
750 mg

A 50-mL bottle of cyclosporine contains 100 mg/mL. If the dose is 7.5 mg/kg, how many milliliters would be administered with each dose to a kidney transplant patient who weighs 158 lb? Round your answer to 1 decimal place
5.4 ml

The recommended pediatric dose of epinephrine for allergic reactions is 0.01 mg/kg. If a physician administered 0.15 mg to a child using this dosing recommendation, what was the weight (in pounds) of this child? Round your answer to a whole number
33 lbs
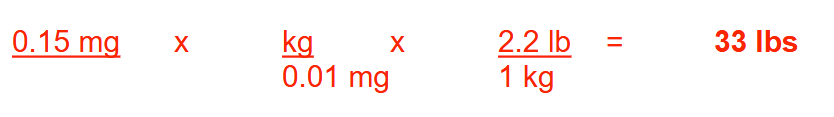
If the pediatric dose of dactinomycin is 15 mcg/kg/day for 5 days, how many milligrams should be administered to a child who weighs 40 lb over the complete course of treatment? Round your answer to 2 decimal places.
1.36 mg
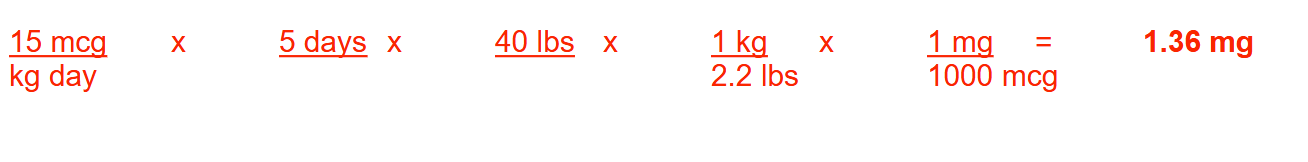
How many grams of codeine sulfate are required to make 5 fl oz of a solution such that each teaspoonful will contain 25 mg of codeine sulfate? Round your answer to 2 decimal places.
0.75 g
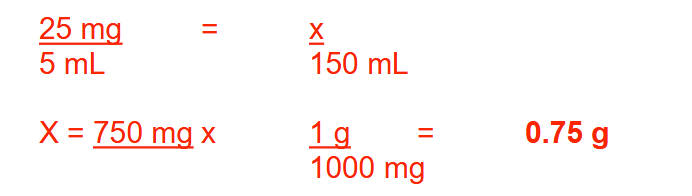
You receive the following prescription to be compounded:
Rx:
Ephedrine sulfate 0.48 g
Aspirin 7.2 g
Caffeine 0.24 g
Divide into 24 caps
Sig: 2 caps PO q6h prn
How many milligrams of ephedrine sulfate will the patient receive in each dose? Round your answer to a whole number
40 mg
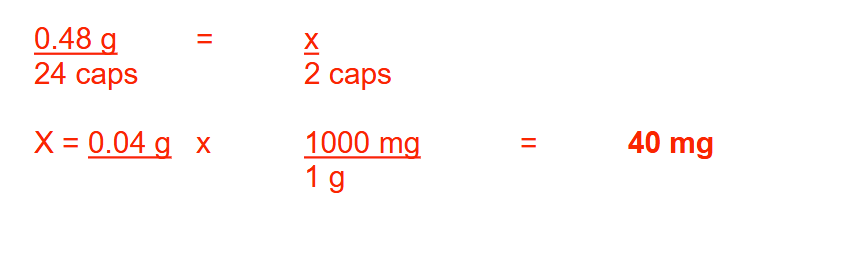
If 500 mL of a 30% (w/v) solution is diluted to 2 gallons, what is the percentage concentration (w/v) of the diluted solution? Round your answer to 2 decimal places
1.98%
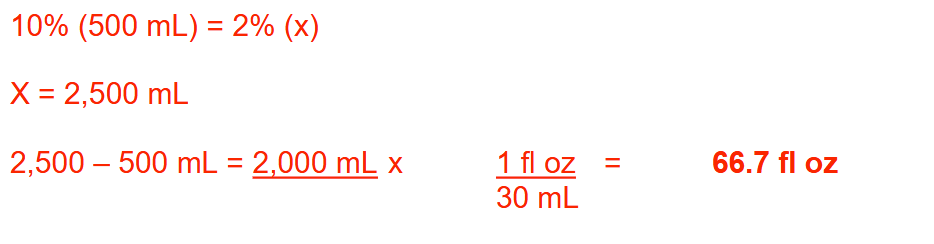
How many fluid ounces of water should be added to 500 mL of 10% (w/v) benzalkonium chloride solution to make a 2% (w/v) solution? Round your answer to 1 decimal place
66.7 fl oz
How many ounces of a 25% (w/w) coal tar ointment are needed to prepare 1 pound of a 10% (w/w) coal tar ointment? Round your answer to 1 decimal place
6.1 oz
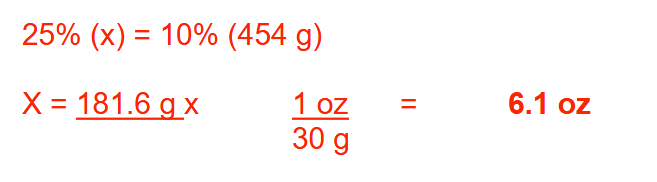
How many milliliters of water should be added to 200 mL of a topical solution containing 1:50 (w/v) methyl salicylate to make a solution containing 1:400 (w/v) methyl salicylate? Round your answer to a whole number.
1400 ml

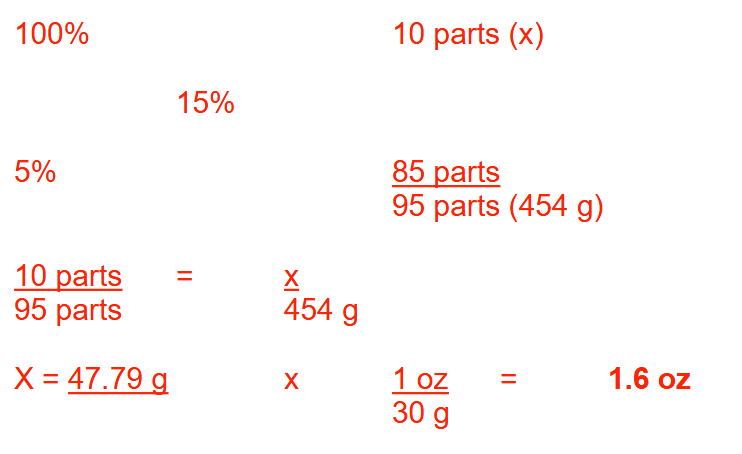
A prescription is written for 454 g of a 15% (w/w) zinc oxide ointment. How many ounces of zinc oxide must be mixed with a 5% (w/w) zinc oxide ointment to prepare this prescription? Round your answer to 1 decimal place
1.6 oz
How many milliliters of a 60% (w/v) stock solution must be used to prepare 3 pints of a 5% (w/v) solution? Round your answer to 2 decimal places
118.25 ml

How many grams of an ointment base should be added to 50 g of a 5% (w/w) coal tar ointment to make a 2% (w/w) coal tar ointment? Round your answer to a whole number
75 g
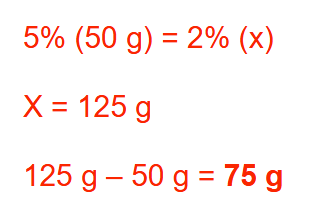
How many ounces of coal tar should be added to 380 g of a 5% (w/w) coal tar ointment to prepare a 20% (w/w) coal tar ointment? Round your answer to 1 decimal place
2.4 oz
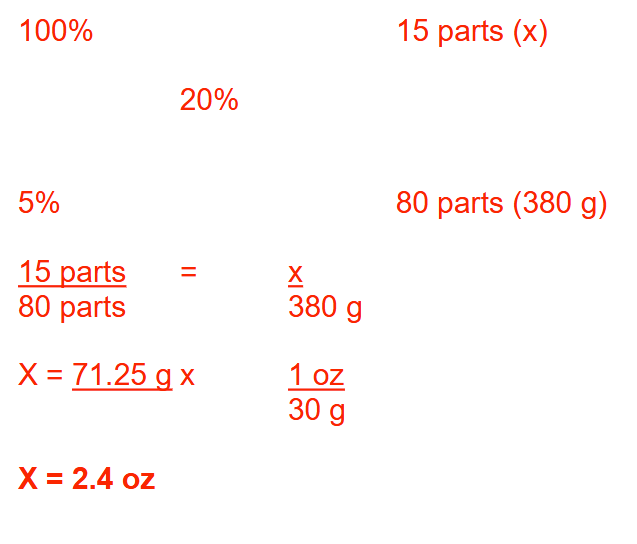
A pharmacist mixes 50 g of 20% (w/w) sulfur ointment, 100 g of 10% (w/w) sulfur ointment, and 25 g of sulfur with 120 g of petrolatum base. What is the percentage strength (w/w) of sulfur in the final product? Round your answer to 1 decimal place.
15.3%
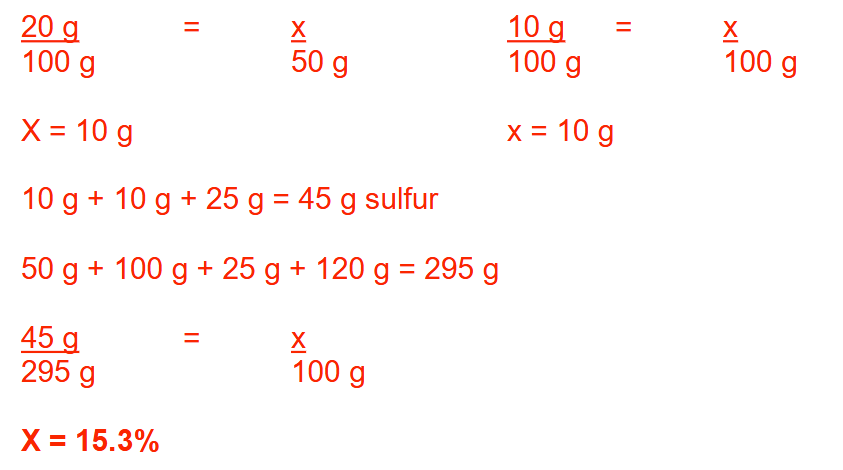
Water is added to 5 fluid ounces of a 1:100 (w/v) epinephrine solution to make 1 pint of this solution. What is the percentage strength (w/v) of epinephrine in the final solution? Round your answer to 2 decimal places.
0.32%
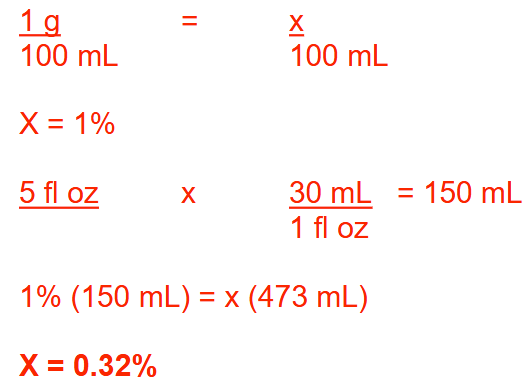
How many grams of hydrocortisone powder should be added to 4 ounces of a 0.1% (w/w) hydrocortisone cream to prepare a 5% (w/w) hydrocortisone cream? Round your answer to 1 decimal place
6.2g

How many milliliters of a phenobarbital elixir containing 25 mg/tsp should be mixed with a phenobarbital elixir containing 35 mg/tsp to make 6 fluid ounces of a phenobarbital elixir containing 6.2 mg/mL? Round your answer to a whole number.
72 ml

How many grams of white petrolatum should be added to a 37.5% (w/w) ichthammol ointment to produce 5 lb of a 10% (w/w) ichthammol ointment? Round your answer to 2 decimal places
1664.67 g
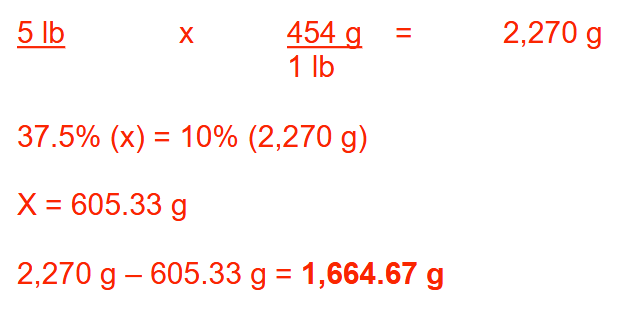
An oral solution contains 4.25% (w/v) of drug. A pharmacist wished to change the drug concentration to 5 mg/mL by using a diluent of polyethylene glycol. How many milliliters of the 4.25% (w/v) oral solution should be used to prepare 3 quarts of the new mixture? Round your answer to 1 decimal place
333.9 ml

You have in your pharmacy 3 ounces of a 12% (w/w) zinc oxide ointment, 135 g of a 10% (w/w) zinc oxide ointment, and 0.75 lb of a 3% (w/w) zinc oxide ointment. What would be the percentage strength of zinc oxide if all three of these ointments are mixed together? Round your answer to 1 decimal place
6.1%

How many milliliters of a 0.9% (w/v) NaCl solution are needed to make the following prescription isotonic? Round your answer to 2 decimal places.
Rx:
Cocaine HCl (E = 0.17) 0.15 g
Sodium chloride qs
Purified water qs ad 15 mL
Make isotonic soln
Sig: One drop in left eye
12.17 ml

How many milliliters of a 5% (w/v) boric acid solution are needed to make the following prescription isotonic? Round your answer to 2 decimal places.
Rx:
Oxymetazoline HCl (E = 0.20) 0.5% (w/v)
Zinc sulfate (E = 0.09) 0.2% (w/v)
Boric acid (E = 0.52) qs
Purified water qs ad 30 mL
Make isotonic soln
Sig: For the nose, as decongestant.
9.02 ml

How many grams of potassium nitrate (E = 0.58) are needed to make the following prescription isotonic? Round your answer to 2 decimal places.
Rx:
Silver nitrate solution 1:500 (w/v) (E = 0.33) 60 mL
Make isotonic solution
Sig: For eye use
0.86 g

How many milligrams of boric acid are needed to make the following prescription isotonic? Round your answer to 1 decimal place.
Rx:
Ephedrine sulfate (E = 0.23) 0.5 g
Boric acid (E = 0.52) qs
Purified water qs ad 50 mL
Make isotonic solution
Sig: 1 gtt in left eye BID
644.2 mg

How many grams of sodium chloride are needed to make the following prescription isotonic? The epinephrine 0.1% solution is already isotonic. Round your answer to 2 decimal places.
Rx:
Tetracaine hydrochloride (E = 0.18) 0.5% (w/v)
Epinephrine 0.1% (w/v) (E = 0.18) 10 mL
Sodium chloride qs
Purified water qs ad 30 mL
0.15 g

How many milliequivalents of potassium will a child receive each day when administered potassium penicillin V (KC16H17N2O5S; MW = 388.5) at a dose of 250 mg PO q6h? Round your answer to 2 decimal places.
2.57 mEq
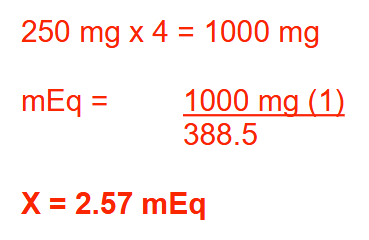
The typical dose of lithium carbonate (MW = 74) in the treatment of bipolar disorder is 600 mg PO TID. How many milliequivalents of lithium will a patient taking this dose receive each day? Round your answer to 1 decimal place
48.7 mEq
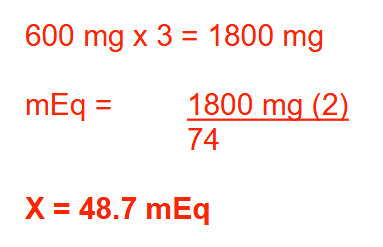
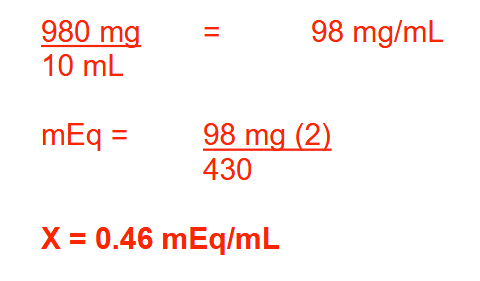
A 10-mL ampule of solution contains 980 mg of calcium gluconate (Ca[C6H11O7]2 ; MW = 430). The specific gravity of the solution is 1.08 g/mL. Calculate the concentration of the solution in mEq/mL. Round your answer to 2 decimal places
0.46 mEq/ml
Rx:
Potassium citrate monohydrate 20% (w/v)
Distilled water qs ad 1 quart
Sig: 15 mL PO TID
(Potassium citrate monohydrate: K3C5H5O7.H2O; MW = 324)
How many milliequivalents of potassium are provided in each dose? Round your answer to 1 decimal place.
27.8 mEq
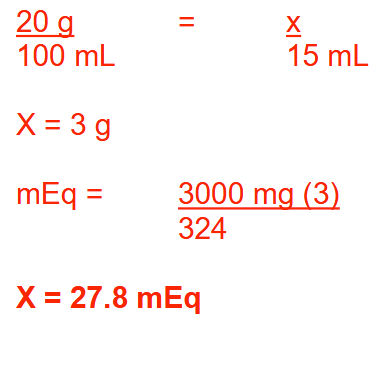
A patient is to receive ammonium chloride at a dose of 36 mg/kg. If the patient weighs 178 lb, how many milliliters of a sterile solution of ammonium chloride (MW = 53.5) containing 0.4 mEq/mL should be administered? Round your answer to 1 decimal place
136.1 ml
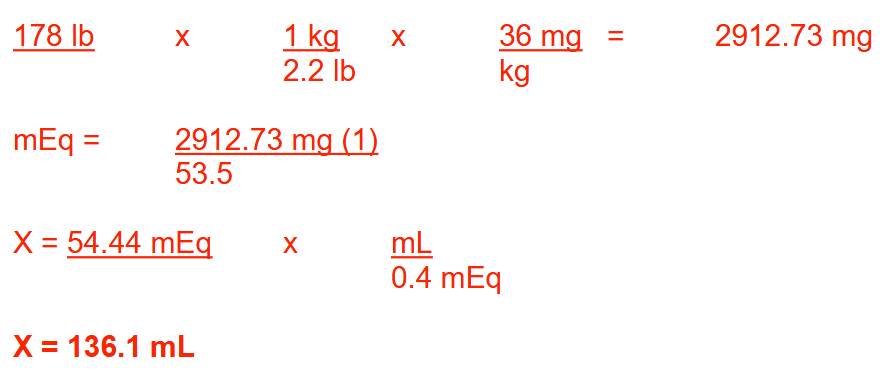
A physician wishes to administer 1.2 million units of penicillin G potassium IV every 4 hours. If 1 unit of penicillin G potassium (C16H17KN2O4S; MW = 372) equals 0.6 mcg, how many milliequivalents of potassium will the patient receive in a 24-hour period? Round your answer to 1 decimal place
11.6 mEq

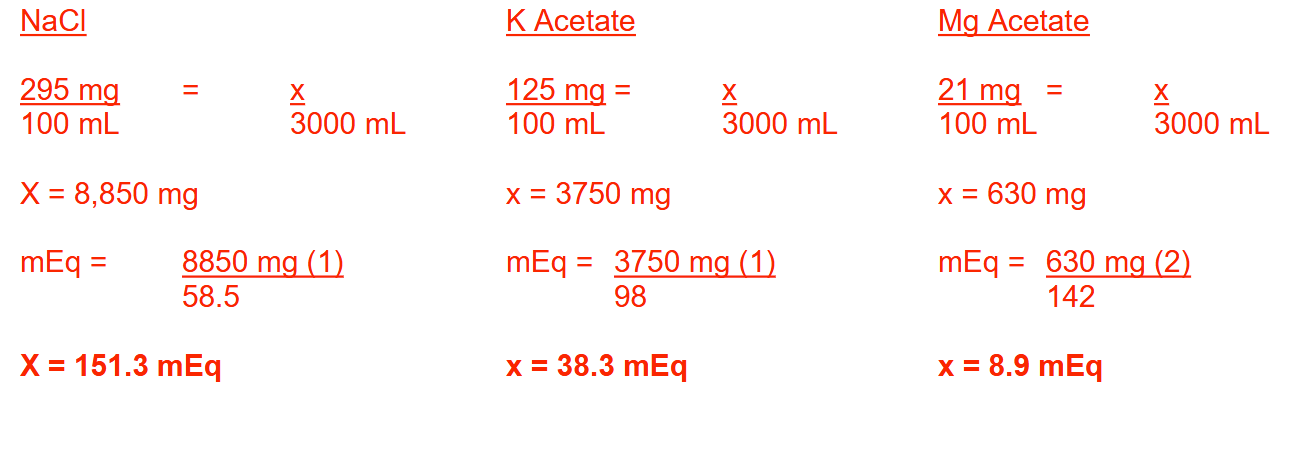
A patient receives 3 liters of the following parenteral fluid:
Sodium chloride 295 mg
Potassium acetate (C 2H 3 KO 2) 125 mg
Magnesium acetate (C 4H 6 MgO 4) 21 mg
Water for injection qs ad 100 mL
How many milliequivalents each of sodium, potassium, and magnesium does the patient receive from this parenteral fluid? Round each answer to 1 decimal place.
Molecular weights:
Na = 23; K = 39; Cl = 35.5; Mg = 24; C = 12; H = 1; O = 16
NaCl = 151.3 mEq
K Acetate = 38.3 mEq
Mg Acetate = 8.9 mEq
How many millimoles of chloride ion are present in 1 teaspoonful of a 10% solution of magnesium chloride (MW: Mg = 24; Cl = 35.5)? Round your answer to 1 decimal place
10.5 mmol
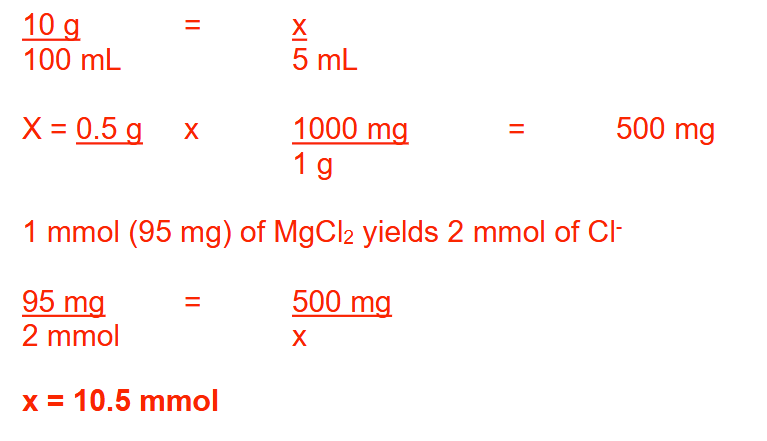
How many grams of potassium carbonate (MW: K = 39; CO3 = 60) will contain the same quantity of potassium as 3.5 g of potassium chloride (MW: K = 39; Cl = 35.5)? Round your answer to 1 decimal place
3.2 g

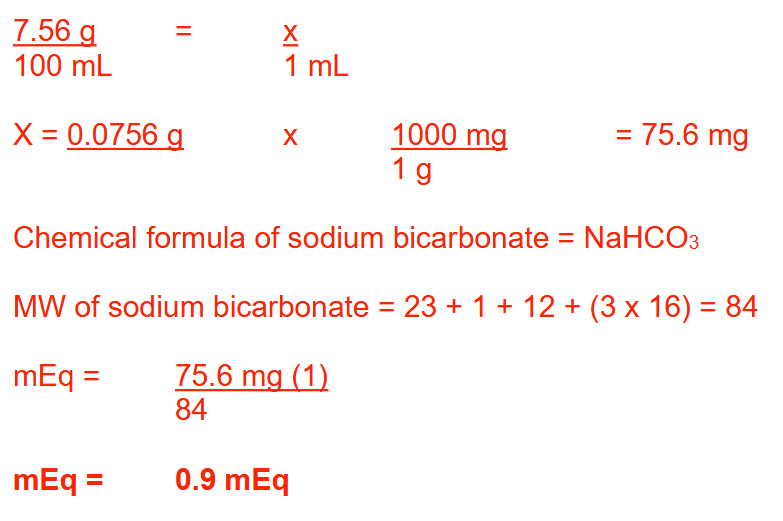
You are asked to prepare a 7.56% solution of sodium bicarbonate (MW: Na = 23; C = 12; H = 1; O = 16). How many milliequivalents of sodium bicarbonate will there be in each milliliter of this solution? Round your answer to 1 decimal place
0.9 mEq
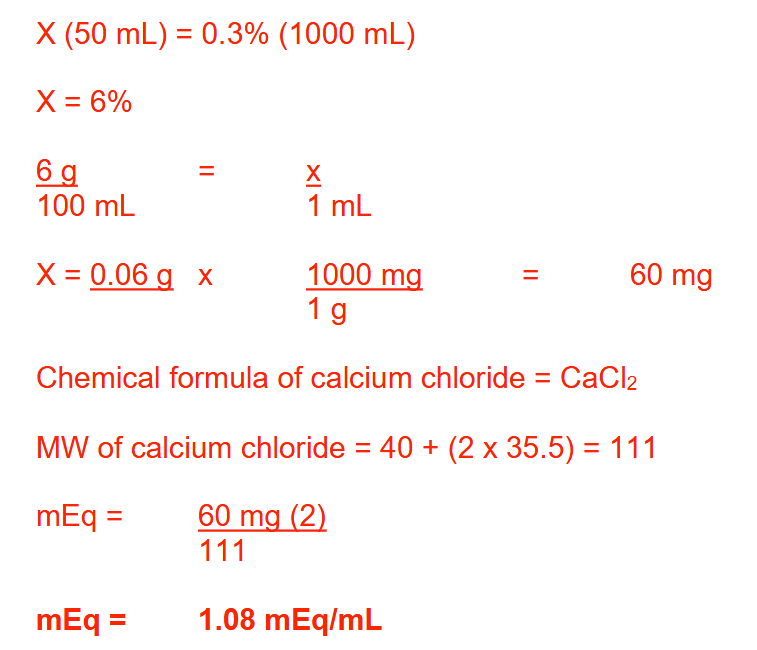
A 50-mL vial of calcium chloride (MW: Ca = 40; Cl = 35.5) was diluted with sterile water for injection to make 1 L of solution. The concentration of calcium chloride in the finished product was 0.3%. What was the concentration, in mEq/mL, of the original solution? Round your answer to 2 decimal places
1.08 mEq/ml
You receive the following prescription to be compounded:
Rx:
Calcium citrate solution
1 tsp (10 mEq) PO 4 times daily x 3 days
The molecular formula of calcium citrate is Ca3(C6H5O7)2; MW = 498
How many grams of calcium citrate will be needed to compound this prescription? Round your answer to 2 decimal places.
9.96 g

A solution is prepared by adding 50 g of calcium chloride (CaCl 2; MW = 111) to enough water to make 500 mL of solution. Express the concentration of the solution as mOsm/L. Round your answer to 1 decimal place
2702.7 mOsm/L

Rx
Potassium citrate monohydrate 20% (w/v)
Distilled water, q.s. 1 quart
Sig: 15 mL po TID
(Potassium citrate monohydrate: K3C5H5O7.H2O; MW = 324)
What is the osmolarity of the solution, expressed as mOsm/L? Round your answer to 1 decimal place
2469.1 mOsm/L
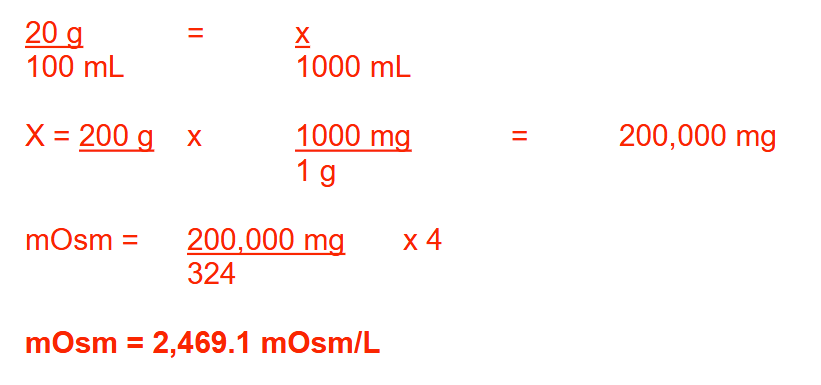
A 10-mL prefilled syringe contains a 4.2% (w/v) solution of sodium bicarbonate (MW: Na = 23; H = 1; C = 12; O = 16). What is the osmolarity (in mOsm/L) of this solution? Round your answer to a whole number
1000 mOsm/L
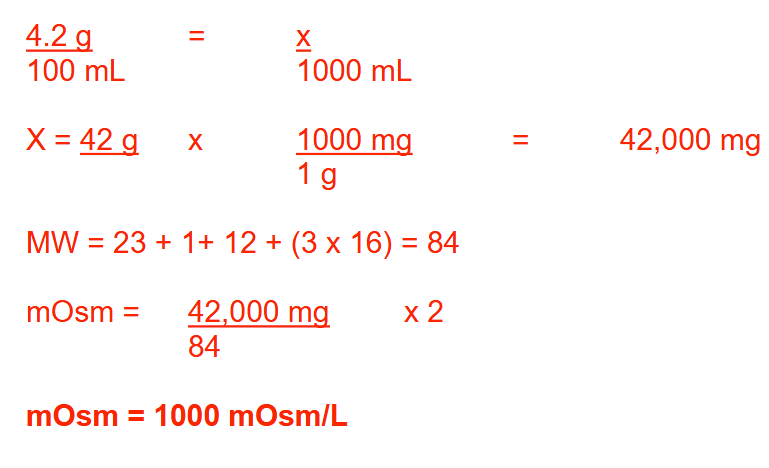
The osmolarity of a potassium chloride solution (MW = 74.5) is 220 mOsm/L. Express this concentration as a percentage strength. Round your answer to 2 decimal places
0.82%
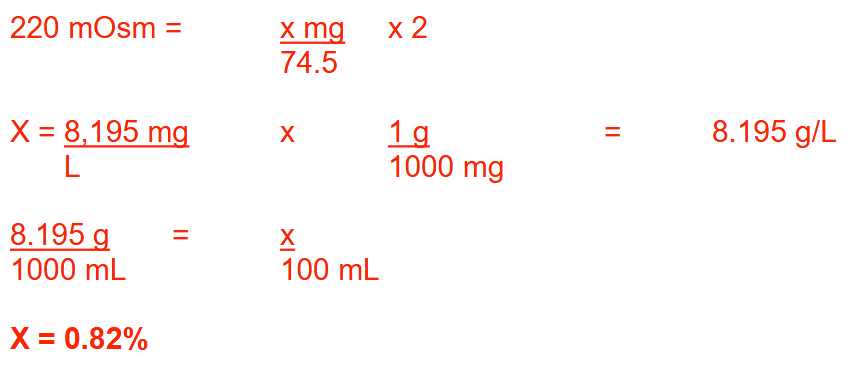
A patient weighing 183 lb is to receive 0.2 mEq/kg of calcium gluconate (Ca [C6H11O7]2 ; MW = 430). How many milliosmoles of calcium gluconate did this patient receive? Round your answer to 2 decimal places
24.95 mOsm

How many grams of CaCl2 · 2H2O (MW: Ca = 40; Cl = 35.5; H = 1; O = 16) should be dissolved in water to make 150 mL of a solution that contains 298 mOsm/L? Round your answer to 2 decimal places
2.19 g
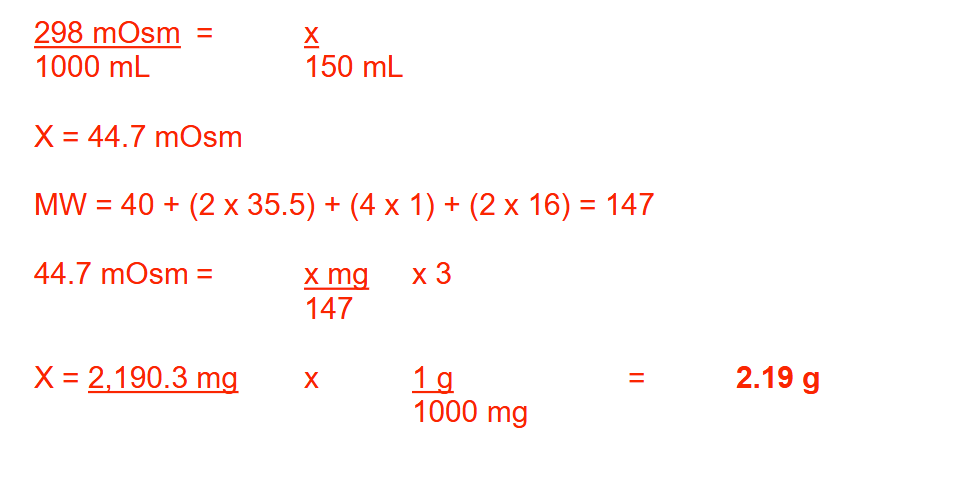
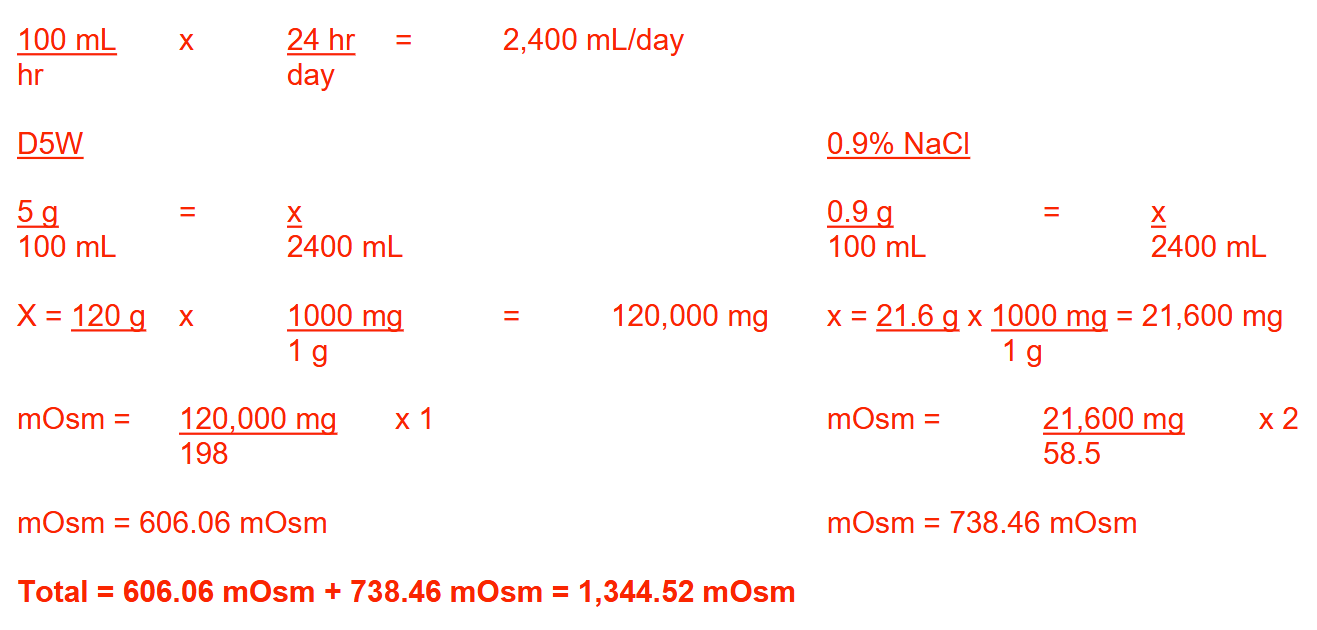
A dehydrated patient is scheduled to receive D5W/0.9%NaCl solution intravenously at a rate of 100 mL/hr. How many milliosmoles will this patient receive in one day? (MW: 5% dextrose, monohydrate = 198; NaCl = 58.5)? Round your answer to 2 decimal places
1344.52 mOsm
A solution contains 20% mannitol (MW = 182) in sterile water for injection. How many mOsm/L are represented by this concentration? Round your answer to 1 decimal place
1098.9 mOsm/L

How many mOsm/L are there in a solution containing 5% dextrose (monohydrate; MW = 198) and 0.2% sodium chloride (MW = 58.5)? Round your answer to the nearest whole number
321 mOsm/L

skip
skip

A patient who weighs 160 lbs is to receive a drug at 50 mcg/kg/hr. If the infusion is to run at 2 mL/minute, how many milligrams of the drug will the patient receive in 3 hours? Round your answer to 1 decimal place
10.9mg
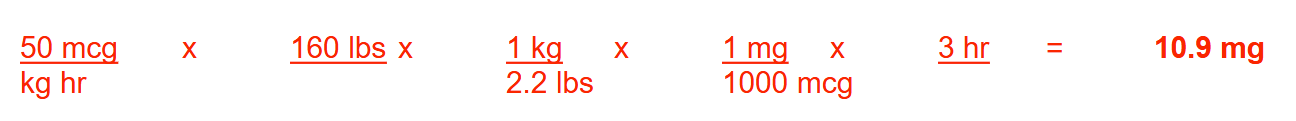
A patient in septic shock who weighs 185 lbs is presently receiving a norepinephrine drip at a rate of 0.5 mcg/kg/min. The infusion is available as 100 mg/250 mL 0.9% NaCl. What should this patient’s infusion rate be in mL/hr? Round your answer to 1 decimal place
6.3ml/hr
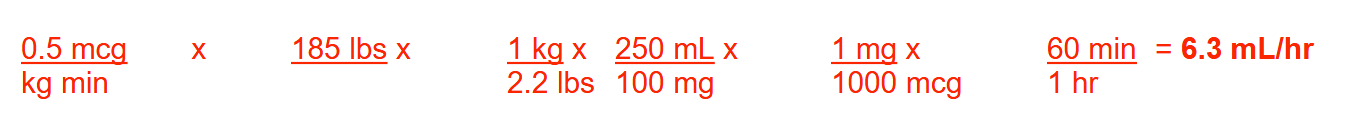
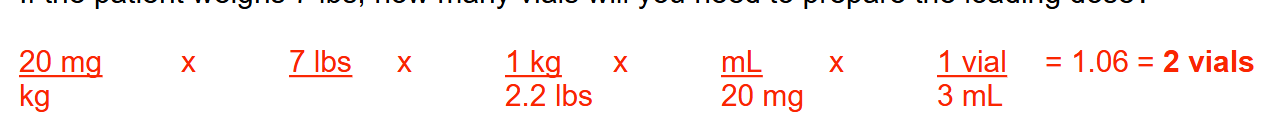
You receive the following order for IV caffeine citrate (loading dose immediately followed by maintenance dose)
Loading dose: 20 mg/kg infused over 30 minutes
Maintenance dose: 5 mg/kg/day infused over 30 minutes
Caffeine citrate vials contain 3 mL and come in a concentration of 20 mg/mL
If the patient weighs 7 lbs, how many vials will you need to prepare the loading dose?
2 vials
Daptomycin 6 mg/kg has been ordered for a patient weighing 214 lbs. A reconstituted vial contains daptomycin at a concentration of 500 mg/10 mL. How many milliliters of the reconstituted drug should be placed in a 0.9% NaCl 50-mL bag to complete the order? Round your answer to 1 decimal place
11.7 mL
