pdh complex and tca cycle
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
PDH complex overview
pyruvate dehydrogenase complex exists in mitochondrial matrix
oxidative-decarboxylation of pyruvate (alpha-ketoacid) converts it to acetyl-CoA
5 coenzymes assisting PDH complex
thiamine pyrophosphate/TPP
lipoamide
NAD+
FAD
reduced Coenzyme A/CoASH
coenzyme
organic cofactor
where is TPP used?
pyruvate dehydrogenase/E1
where are lipoamide and CoASH used?
dihydrolipoamide transacetylase/E2
where are NAD+ and FAD used?
dihydrolipoamide dehydrogenase/E3
alpha-ketoacid dehydrogenase complex arrangement
three enzyme complexes: pyruvate dehydrogenase, alpha-ketoglutarate dehydrogenase, alpha-ketoacid dehydrogenase → all oxidative decarboxylation reactions, utilize complex
E1: binds TPP, catalyzes decarboxylation
E2: transfers TPP bound ketoacid to thiol groups of lipoamide, adds CoA to ketoacid
E3: reduces FAD then reduces NAD+, using electrons obtained from reduction of lipoamide
thiamine pyrophosphate/TPP
stabilizes carbanion transition state
forms covalent adduct with pyruvate, provides e- delocalization
facilitates cleavage of C-C bonds by stabilizing carbanion intermediates
C2 on ring, between N and S, is deprotonated to form nucleophilic carbanion → attacks carbonyl carbon of pyruvate → ring stabilizes intermediate through resonance
tightly bound to E1
lipoamide
transfers acyl groups in alpha-keotacid dehydrogenases
has “swinging arm”: side chain in an E2 domain, interacts with active sites of E1/E3
accepts aldehyde fragment from TPP via oxidation of aldehyde and reduction of lipoamides disulfide group at the same time → generates acyl group which is transferred to CoA, pair of e- donated to lipoamide creates dihydrolipoamide
acts as e- carrier and an acyl group carrier
covalently bound to E2 by amide bond linking carboxyl of lipoid acid to lysine amino
coenzyme A/CoA
activates acyl groups (ex.: acetyl group from pyruvate)
acylation requires energy input: comes from oxidative decarboxylation of pyruvate
acylated = acyl-SCoA, unacylated = HS-CoA
acetyl-CoA is a thioester → high chemical potential due to resonance stabilization
have C-SR instead of C-OR, larger size of S destabilizes bond = higher potential energy of acyl group transfer compared to an oxygen ester = more favorable deltaG of hydrolysis
C-SR thioester bond is weaker than C-OR bond of esters, R-S- is good leaving group → acyl group is readily transferred
flavin adenine dinucleotide/FAD
derived from riboflavin/vitamin B
contains isoalloxazine ring system = 2 electron acceptor → ring attached to ribitol, ribitol attached to adenosine via pyrophosphate link
riboflavin: ring attached to ribitol
flavin mononucleotide: ring attached to ribitol attached to phosphate
undergoes two-electron oxidation and reduction reactions
tightly bound to E3: coenzyme cannot easily dissociate from enzyme → flavins cannot transfer electrons by moving from enzyme to enzyme
nicotinamide adenine dinucleotide/NAD+
electron carrier, participates in reversible two electron redox reactions via hydride transfers
hydride transfer: both hydrogen and electron pair are transferred
substrate is oxidized → reduced NADH forms, leaves enzyme, is reoxidized by other systems
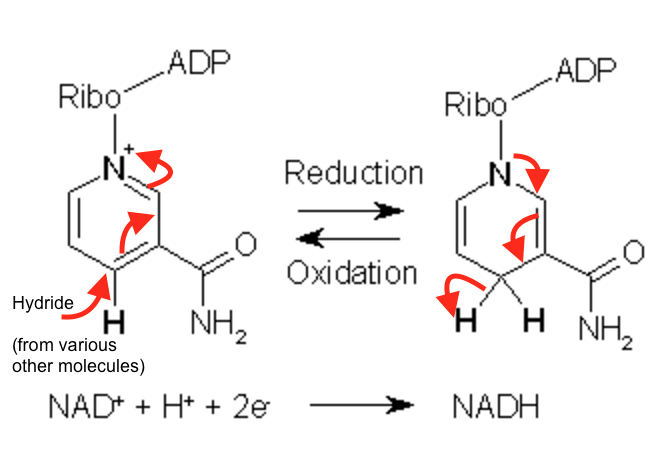
NAD+ and NADH redox
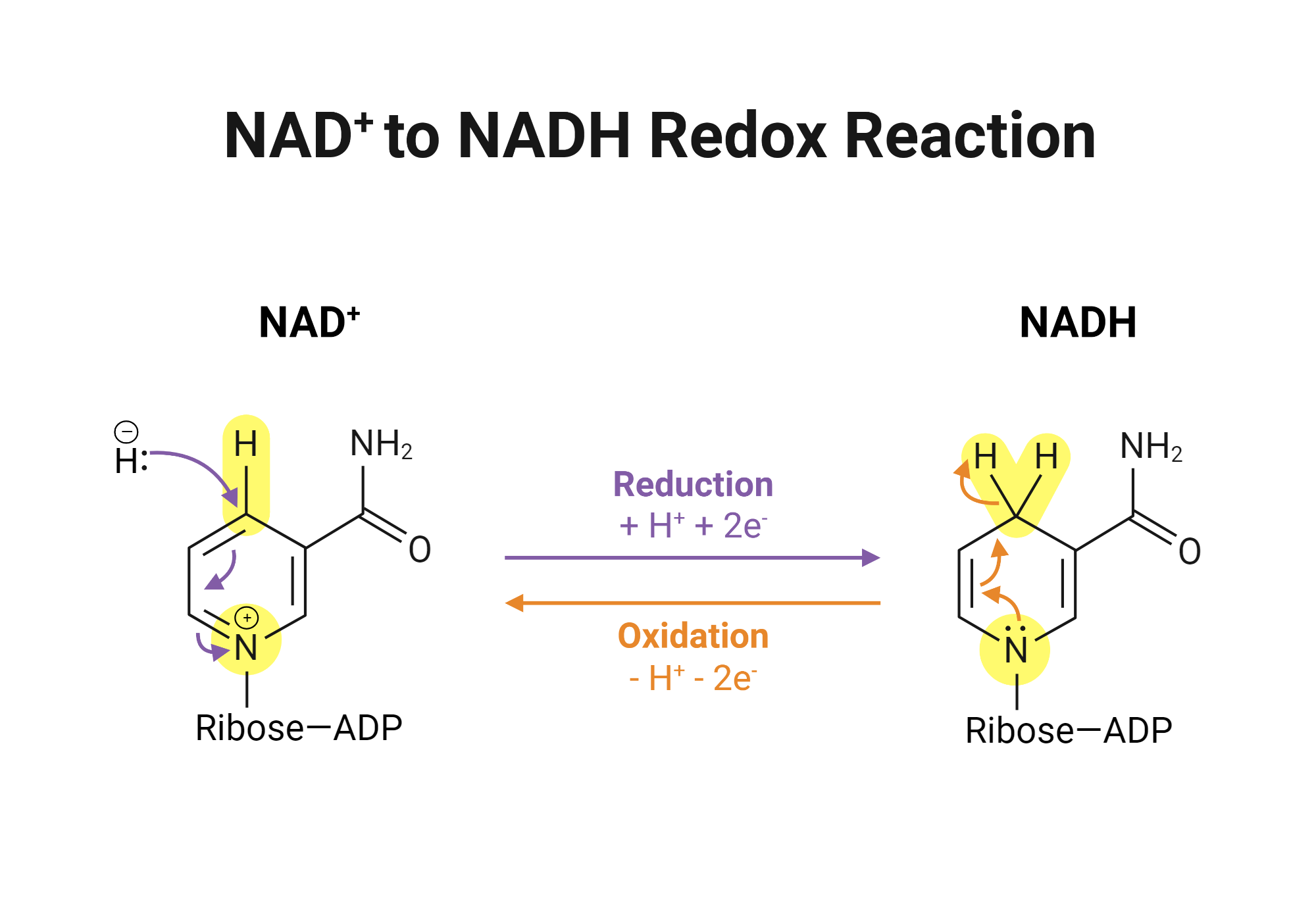
NAD+ and NADH redox
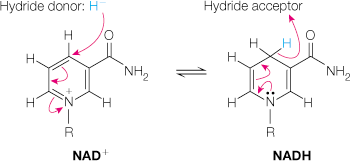
NAD+ and NADH redox
arsenic poisoning
arsenite inhibits all enzymes requiring lipoamide as a cofactor, thus E2 is affected and inhibited
arsenite forms complex with thiol/-SH groups of lipoamide and thus makes it unavailable as a cofactor (covalently modifies lipoamide groups)
pyruvate cannot be efficiently converted to acetyl-CoA, pyruvate accumulates, lactate accumulates, respiration cannot proceed
TCA overview
acetyl + 4 carbon oxaloacetate = 6 carbon citrate
rearranged into isocitrate
oxidative decarboxylation = e- transferred to NAD+ to form NADH/H+, CO2 released, ATP generated
succinate oxidized to oxaloacetate, FADH2 generated, NADH/H+ generated
citrate synthase
condenses activated acetyl group with oxaloacetate to form citrate
aconitase
rearranges OH on citrate to form isocitrate
requires Fe2+
isocitrate dehydrogenase
RATE LIMITING ENZYME
catalyzes oxidative decarboxylation of isocitrate: transfers electrons to NAD+ to generate NADH/H+, releases one carboxylate (COO-) as CO2
alpha-ketoglutarate dehydrogenase complex
structurally/functionally similar to PDH complex, requires same cofactors
causes second oxidative decarboxylation: removes one carboxylate (COO-) group as CO2, transfers electrons to NAD+ to generate NADH/H+, condenses CoASH to generate succinyl-CoA
succinyl-CoA synthetase
energy from succinyl-CoA thioester bond generates ATP from ADP + Pi.
substrate-level phosphorylation
succinate dehydrogenase
transfers pair of electrons from acetyl group to FAD to generate FADH2 and fumarate
resides in inner mitochondrial membrane, unlike all others in mitochondrial matrix
fumarase
incorporates H2O into fumarate to generate l-malate
l-malate dehydrogenase
transfers final electron pair from acetyl group to NAD+ to generate NADH/H+ and regenerate oxaloacetate
3 stages of respiration
1: generation of acetyl-CoA + two electrons
mitochondrial matrix
2: oxidation of acetyl-CoA into 2 CO2 + 8 electrons, NADH + FADH2
mitochondrial matrix
3: reoxidation of reduced e- carries provides energy for ATP synthesis, NAD+ + FAD
mitochondrial inner membrane
oxidation and reduction
oxidation removes e- from substrate (donor) and gives to carrier (acceptor)
metabolic oxidations = loss of hydrogen from substrate → catalyzed by dehydrogenases
reduction gives e- to substrate
dicarboxylic acids: citrate, succinate, fumarate, malate
act catalytically
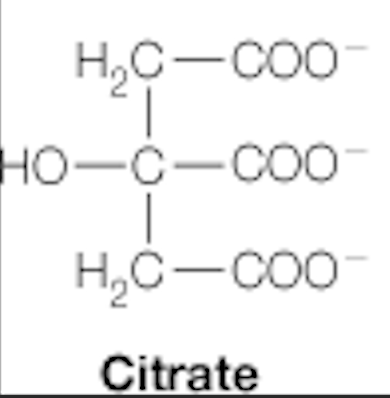
citrate
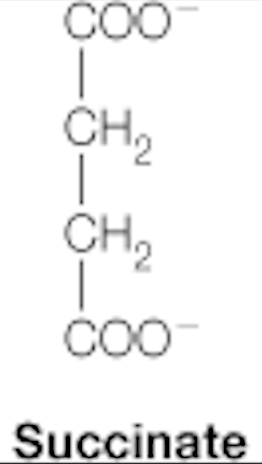
succinate
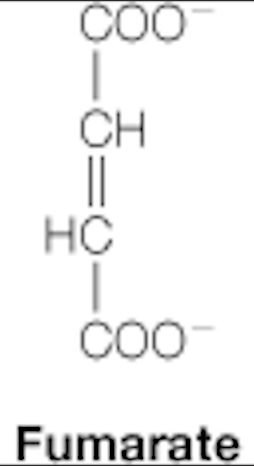
fumarate
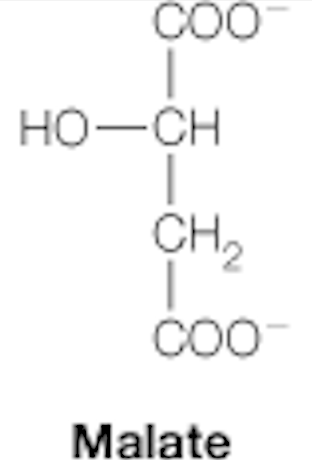
malate
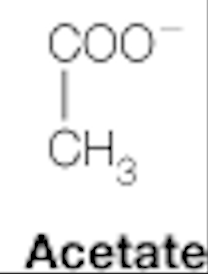
acetate
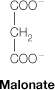
malonate
TCA cycle regulation
corresponds to rate of electron-transport chain → regulated by ATP/ADP ratio and rate of ATP use
messengers: ADP, NADH
increased ADP activates, increased NADH inhibits
isocitrate dehydrogenase = principle enzyme regulator → activated by increased ADP/Ca2+, inhibited by increased NADH
alpha-ketoglutarate dehydrogenase complex: activated by Ca2+, inhibited by NADH
malate dehydrogenase: inhibited by NADH
citrate synthase: inhibited by high citrate concentrations
TCA cycle intermediates
malate: produced in liver during fasting from glucogenic precursors, leaves mitochondria for cytosolic gluconeogenesis
liver uses other intermediates to synthesis carbon skeletons of AA’s
succinyl-CoA: may be removed to form heme in liver, bone marrow
alpha-ketoglutarate: converted to glutamate in brain, then to GABA
alpha-ketoglutarate: converted to glutamate in skeletal muscle, transported through body