Molecular Biology of Cancer 1: How do cancers arise?
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
How do cancer cells arise even with DNA repair mechanisms?
Genomic DNA is constantly under attack, cells have repair mechanisms but if these fail, DNA damage can accumulate
Cells with damaged DNA can undergo apoptotic cell death, but if it fails it can lead to the development of a cancer cell
Both _________ and _________ agents can cause mutations to genomic DNA.
exogenous (within cell), exogenous (outside of the cell)
What are some endogenous causes of DNA damage?
Errors in replication (1 in 10^7)
Introduced by polymerases, in most cases are repaired
Reactive oxygen species (ROS)
By products of metabolism can modify nucleotides + bases
What are some exogenous causes of DNA damage?
• Ultraviolet [UV 200-300nm] radiation from the sun
• Other radiation - x-rays and gamma rays
• Mutagenic chemicals e.g., DNA intercalating agents
• Cancer chemotherapy and radiotherapy
Kill cells by inducing DNA damage, normal cells overcome (Repair), cancer cells cannot
Different DNA damaging agents can give rise to different forms of damage (Or types of mutations), what are some possible forms?
2 Principle Outcomes:
Block replication machinery, so genome is not replicated how it should be
Double strand break
Single strand breaks
Pyrimidine dimers- two adjacent pyrimidine nucleotides become joined
Altered bases (8 oxo guanine lesion)
Alter the DNA sequence, which will produce abnormally coded protein
Insertions
Deletions
Mismatched bases
**DIfferent DNA damaging agents can cause different types of damage which causes different DNA lesions.
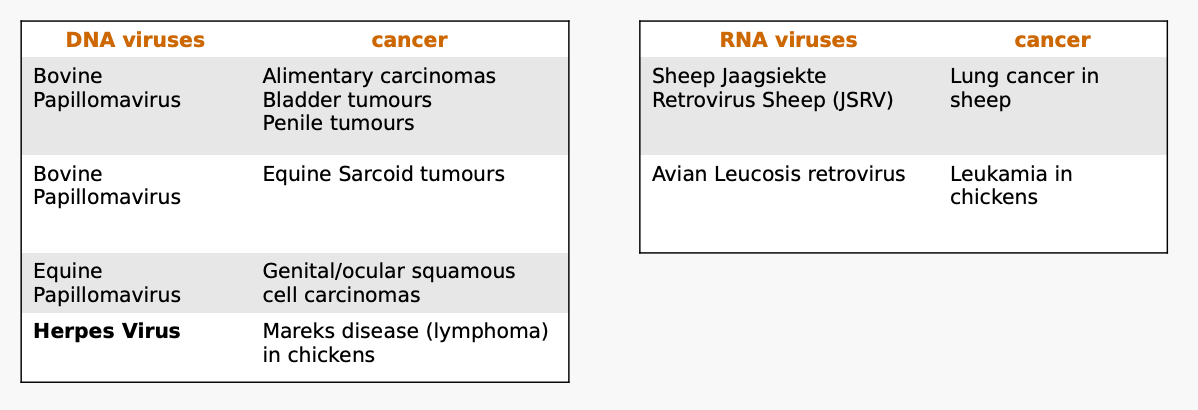
Several viruses are able to induce cancers in animals such as?
Some transmissible cancers exist, what are these caused by?
Transmissible cancer are caused by living cancer cells that are transmitted from animal to animal and are distinct from cancers caused by infectious agents (viruses)
No infectious agent - are the tumor cells themselves
What are some examples of transmissible cancers?
Canine Transmissable Veneral tumour
Causes tumours on the external genitalia
Mainly transmitted by coitus
Biting, licking can also transfer tumours to other body sites
Genetic studies indicate it arose in somatic cells of a dog thousands of year ago- ancient in origin ~11,000 years old
Devil Faced Tumour Disease in Tasmanian devils DFTD
Characterised by tumours on the face neck and mouth
Animals die within a few months
Responsible for widespread decline in devil population; Poses a threat to the survival of species
Cancer is primarily a _____ disease that is _____ in nature.
genetic, clonal
How does cancer usually start?
Usually starts when a single cell acquires a series of mutations, usually one after another, that collectively change a once-normal cell into a cancerous cell that divides uncontrollably
Genetic changes can be as subtle as _____ _____ or as _____ _______ _____.
point mutations, gross chromosomal losses
How many mutations are required for cancer to normally develop?
3 - 7 mutations, this is known as the multi-hit hypothesis
What is the multi-hit hypothesis?
The multi-hit hypothesis of cancer proposes that cancer development typically requires a series of genetic mutations (or "hits") to accumulate in a cell, leading to uncontrolled growth.
Cancers require step-wise accumulation of mutations which occur overtime
How does age correlate with cancer?
Cancer incidence increases with age in humans and animals, mutations accumulate over time
What are tumour suppressor genes?
Genes that act to inhibit the cell cycle, blocking proliferation and tumour development
Apply “brakes” to the cell cycle
Why are mutations to TSG often termed "loss of function”?
Abolish the inhibitory function = loss of function mutation
What are some examples of TSGs?
pRb
p53
BRCA 1/2
What is one of the most common genetic alterations detected in cancers?
TSG frequently mutated
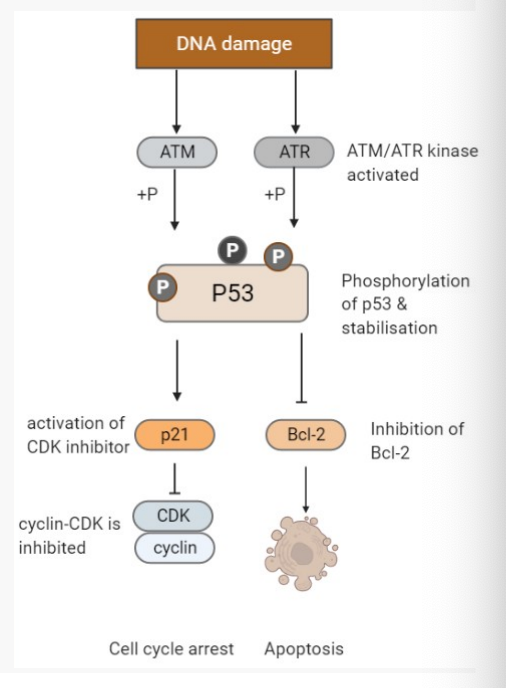
Describe the role of the p53 tumour suppressor in response to DNA damage.
DNA damage occurs
Then… kinase enzymes (ATM and / or ATR ) are activated
Kinase activity causes phosphorylation of p53, this stabilizes the otherwise unstable p53 protein
p53 then functions to trigger cell cycle arrest or apoptosis
Cell cycle arrest is activated by blocking CDK-cyclin function in G1, allows for DNA repair
Apoptosis is triggered by blocking Bcl-2, a key trigger for inducing apoptosis
What happens if the p53 protein function is lost, and what is it’s prevalence in cancer?
• >80% of cancers (humans, animals) have mutated p53 protein hence no functional p53
If p53 function is lost then the cell is unable to trigger a halt of the cell cycle or apoptosis
Cell with mutation can then proceed through the cell cycle
Mutations will be copied to daughter cells; leading to genomic instability meaning more mutations can be acquired
Are there any other ways that the p53 function can be lost (other than cancer)?
• Note: p53 function can also be 'lost' via non mutational mechanisms
Some viruses e.g. Human Papillomaviruses (16/18) that cause cervical cancer in humans can degrade p53 protein
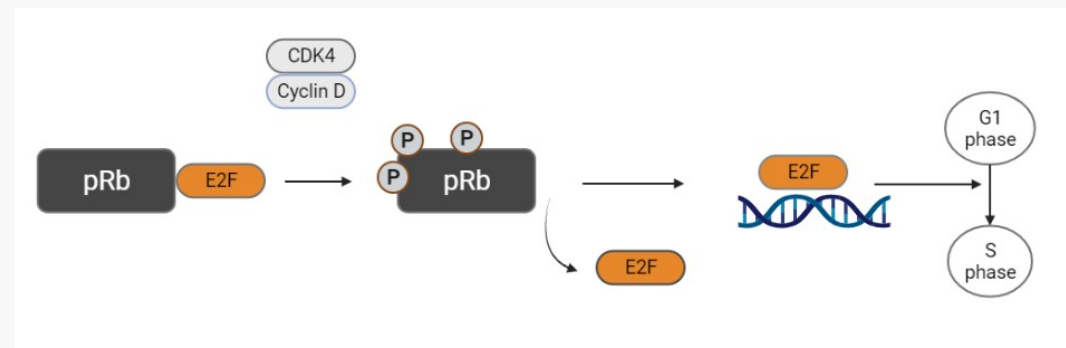
Describe the role of the pRb tumour suppressor in response to DNA damage.
• pRb gene encodes the retinoblastoma protein (pRb)
• Normal pRb protein prevents G1/S phase progression; a critical control of cell proliferation
What happens if pRB becomes mutated?
• Mutated pRB is stimulatory (remains phosphorylated); control on cell cycle is lost; signal for cell to go forward into S phase (DNA synthesis) is always “on”
When pRb is phosphorylated by CDK/cyclin, E2F dissociates and triggers S phase transition from G1
What are proto-oncogenes?
What happens / what are they termed if they become activated/mutation?
Proto-oncogenes are normal genes that function to regulate cell growth; Mutation of a proto-oncogene results in an oncogene
Oncogenes promote cell proliferation- 'gas' pedals, hence mutations in oncogenes are termed 'gain of function' mutations
(Proto-oncogenes are the same gene but not activated, once activated becomes a oncogene…)
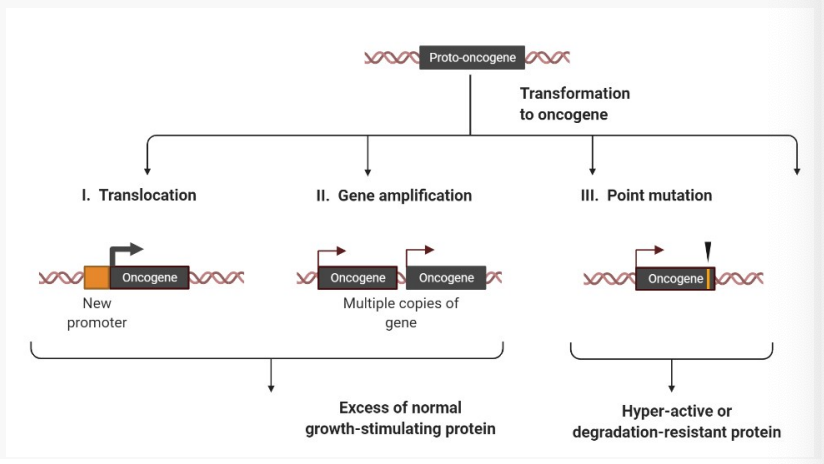
What three mechanisms activate oncogenes?
Oncogenes are activated by 3 mechanisms
1.) Translocation - places the gene under control of a strong gene promoter (regulator)
Breaks in two chromosomes, which join up in the incorrect location.
Results in excessive gene expression.
2.) Gene amplification- numerous copies of the gene results in more protein produced
3.) Point mutation- produces a protein with enhanced/increased function
What are some examples of translocation and what role does it play in cancer pathogenesis?
Ex. Burkitts Lymphoma
Translocation involves immunoglobulin (IgH) gene (chromosome 14) and c-myc proto-oncogene locus (chromosome 8)
Translocation places c-myc under the control of a new strong promoter (IgH promoter), resulting in higher expression levels of c- myc
Downstream of a strong promoter, which transcripts + translates, producing excessive proteins
Ex. Chronic Myelogenous Leukaemia
Abl proto-oncogene inserts into the bcr gene, two genes fuse, the altered abl genes functions improperly, resulting in CML
What is gene amplification and what role does it play in cancer pathogenesis?
Gene amplification increases the number of copies of gene through repeated duplications which results in increased protein levels
Normal cells have a designated number of receptors, if they undergo amplification, number of receptors increase → Excessive protein prod.
What is point mutation and what role does it play in cancer pathogenesis?
Point mutation/deletion due to mutation results in expression of mutant oncoprotein with an enhanced function often called 'hyperactive'
Oncogenes mutations are 'gain of function' mutations (in this instance stronger activity)