Year 10 Chemistry (i'm cooked)
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
78 Terms
What is the structure of an atom?
Nucleus at the centre
Contains protons (positive charge)
Contains neutrons (no charge)
Dense and small compared to the whole atom
Electrons orbit the nucleus
Negative charge
Found in electron shells
Very light compared to protons and neutrons
Most of the atom is empty space
Overall atom is electrically neutral (same number of protons and electrons)
Atomic number = number of protons
Mass number = protons + neutrons
What is the electron shell configuration?
2, 8, 8, 18
What is an element?
An element is a pure substance made of only one type of atom. It cannot be broken down into a simpler substance by chemical means.
How to calculate protons, neutrons, and electrons?
Protons = Atomic Number
Electrons = Protons (if neutral atom)
Electrons = Protons ± charge (for ions)
Neutrons = Mass Number − Atomic Number
What is the periodic table arranged by?
Increasing atomic number (number of protons).
What are groups in the periodic table?
Vertical columns (numbered 1 to 18).
What do elements in the same group have in common?
The same number of valence electrons and similar chemical properties.
What are valence electrons?
Electrons in the outermost shell of an atom.
Why are valence electrons important?
They determine how an atom reacts and bonds with other atoms.
What are periods in the periodic table?
Horizontal rows (numbered 1 to 7).
What do elements in the same period have in common?
The same number of occupied electron shells.
Where are the transition metals located?
In the centre of the table — groups 3 to 12.
What are some properties of transition metals?
Good conductors, high melting points, form coloured compounds, and have variable oxidation states.
What are the names of some important groups?
Group 1: Alkali metals
Group 2: Alkaline earth metals
Group 17: Halogens
Group 18: Noble gases
What formula is used to calculate the maximum number of electrons in an electron shell?
2n², where n is the shell number.
E.g. 2×3² = 18
Define valency
The number of electrons in an atom’s outer shell that an atom can lose, gain, or share to form bonds.
How is an element’s group related to its valency?
For main groups, the valency usually equals the group number (for Groups 1–2 and 13–18).
What group do elements with 8 valence electrons usually belong to?
Group 18 (noble gases).
Why don’t transition metals follow the valency-group rule clearly?
Because they have variable valency and can lose different numbers of electrons.
If an element has 3 occupied electron shells, what period is it in?
Period 3.
Does the period number tell you anything about valence electrons?
No, it only shows the number of electron shells occupied.
What is electron configuration?
The arrangement of electrons in an atom’s shells, listed by the number of electrons in each shell.
How do you predict the charge of an ion?
By looking at how many electrons an atom gains or loses to have a full outer shell.
Metals form cations
Non-metals form annions
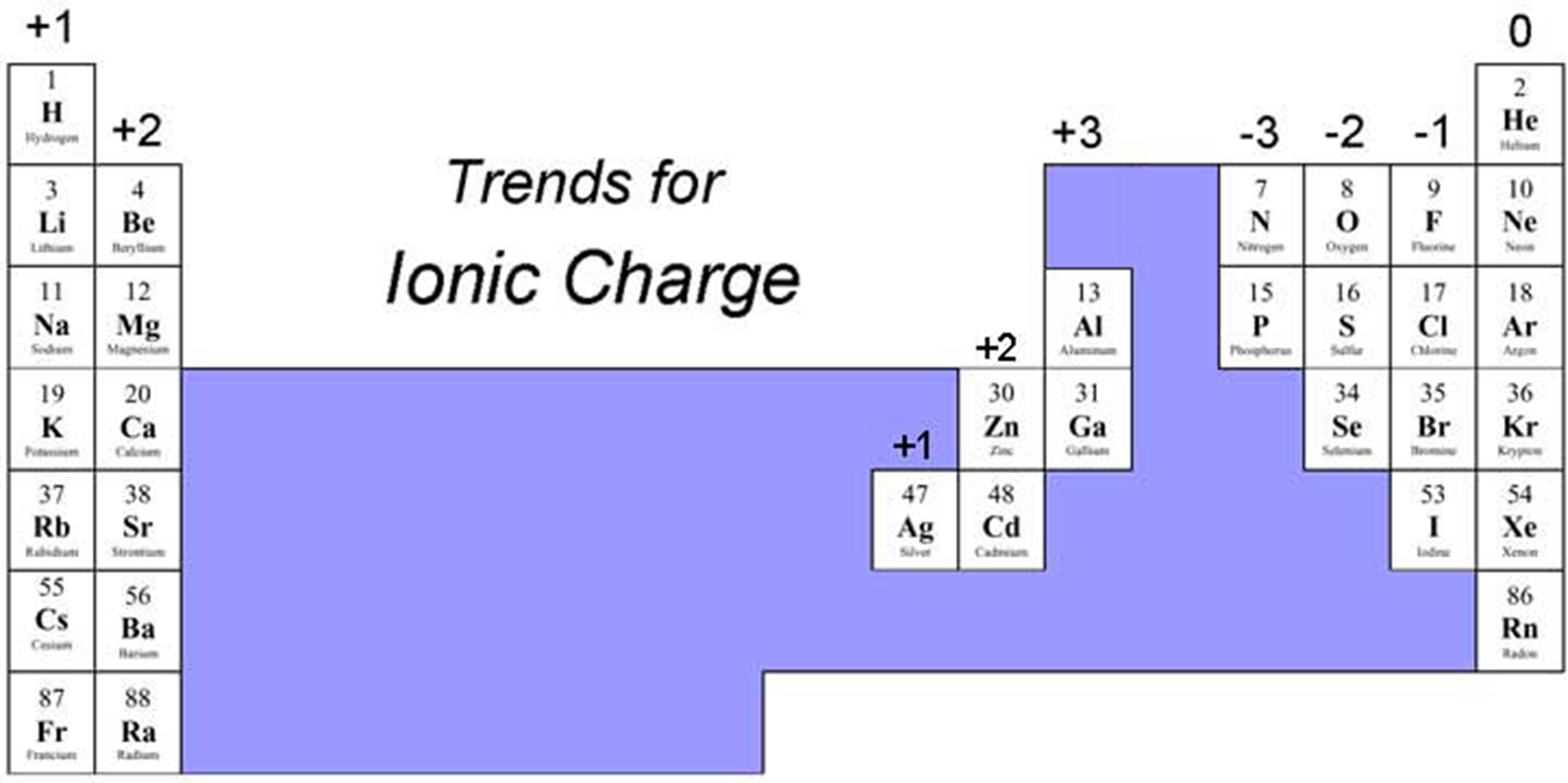
What charge does an atom with 1 valence electron usually form?
+1 (loses 1 electron).
It loses that 1 electron to get a full outer shell underneath, resulting in a positive charge.
What charge does an atom with 7 valence electrons usually form?
-1 (gains 1 electron).
What charge do noble gases usually form?
0 (they are stable and don’t usually form ions).
How do transition metals differ in ion charges?
They can have variable charges, so you often need to check the specific element.
What does diatomic mean?
It means an element naturally forms molecules with two atoms, like H₂.
Why is hydrogen diatomic?
A single hydrogen atom is unstable with only one electron
Two hydrogen atoms share electrons by forming a single covalent bond (H₂)
Makes both atoms stable with a full outer shell.
Only as a pure element or in covalent bonding with itself
Which elements are diatomic?
H₂, N₂, F₂, O₂ I₂, Cl₂, Br₂ — "Have No Fear Of Ice Cold Beer".
Metals vs Non Metals
Feature | Metals | Non-metals |
|---|---|---|
Location on PT | Left and middle | Right side (plus H) |
Appearance | Shiny (lustrous) | Dull or colourful |
Conductivity | Good conductors (heat + electricity) | Poor conductors |
Malleability | Malleable and ductile | Brittle |
State at room temp | Mostly solid (except Hg) | Solid, liquid, or gas |
Example elements | Na, Fe, Cu, Mg, Al | O, N, Cl, S, C, H |
How do ions form?
Ions are formed when an atom gains or loses electrons, resulting in a net electrical charge.
Cation vs Anion
Cation: Positive
Anion: Negative
What are the properties of alkali metals (Group 1)?
Soft, shiny metals
Very reactive (especially with water)
Low melting points
Stored in oil
Reactivity increases down the group
What are the properties of alkaline earth metals (Group 2)?
Harder than alkali metals
Reactive (but less than Group 1)
Burn with colourful flames
Form basic (alkaline) oxides
What are the properties of halogens (Group 17)?
Very reactive non-metals
Poisonous
Coloured
Exist as diatomic molecules (e.g. Cl₂)
Reactivity decreases down the group
What are the properties of noble gases (Group 18)?
Very unreactive
Colourless gases
Full outer electron shells
Low boiling points
Used in lights and signs
Describe the increasing reactivity of metals down Group 1 & 2.
Reactivity increases as you go down the group
Outer electrons are further from the nucleus
Weaker attraction between nucleus and outer electrons
Electrons are easier to lose, so reactions happen more easily
Group 1 metals react more strongly with water than Group 2 metals
What is an isotope?
Atoms of the same element (same number of protons)
Different number of neutrons
Different mass number but same chemical properties
Why do elements form bonds?
The atoms of elements that are unstable are able to fill the outer shells and become stable.
What are the three types of bonding?
Ionic Bonding
Occurs between metal and non-metal atoms
Covalent Bonding
Occurs between non-metals only
Metallic Bonding
Occurs between metal atoms only
All bonds involve electrons and all bonding involve changes to the number of electrons in the outer shells of atoms.
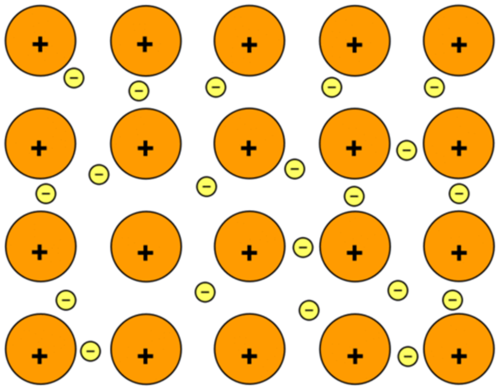
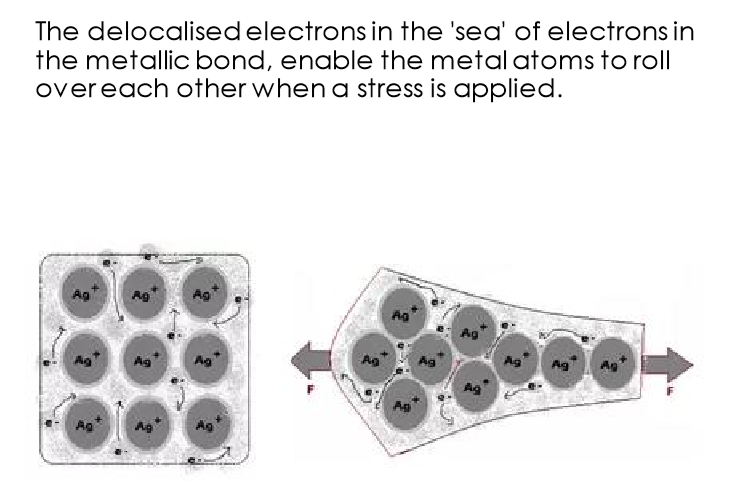
Explain delocalised electrons
In metals, the metal atoms lose their outer electrons to form metal ions
The electrons from all the metal atoms form a “sea” of electrons that can flow around the metal
Delocalised means the electrons are not fixed in one place
electrons hold metal ions in place in a lattice
What are the metallic properties?
Conduct heat
Conduct electricity
Generally high melting and boiling points
Strong (not brittle)
Malleable (can be hammered or pressed out of a shape without breaking)
Ductile (able to be drawn into a wire)
Metallic lustre (shiny)
Opaque (reflect light)
Why does metal have a high melting and boiling point?
The strength of the electrostatic force of attraction (metallic bond)
The bond is very strong and requires large amounts of thermal energy to break
What is an ionic bond?
A bond formed by the transfer of electrons from one atom to another.
Metal and non-metal atoms
Electrons are transferred from each metal atom to each non-metal ions
Metal and non-metal atoms form ions with completely full outer shells and become stable
What happens to the metal atom in an ionic bond?
It loses electrons and becomes a positively charged ion (cation).
What happens to the non-metal atom in an ionic bond?
It gains electrons and becomes a negatively charged ion (anion).
What causes the ionic bond to hold the atoms together?
The positive and negative ions are strongly attracted to each other. This electrostatic attraction is called ionic bonding.
What are compounds that contain ions called?
Ionic compounds
Usually formed by a reaction between a metal and non-metal
What are bonds?
Bonds are forces that hold atoms together.
Types:
Covalent – atoms share electrons (non-metals).
Ionic – atoms transfer electrons (metal + non-metal).
Metallic – free-moving electrons shared among metal atoms.
What are the properties of ionic bonds?
High melting & boiling points
Conduct electricity when molten or in solution
Usually solid at room temperature
Form crystals
Soluble in water
Brittle
Describe the structure of covalent bonds
Covalent bonds have a structure where atoms share electrons to fill their outer shell.
The shared electrons join the atoms together.
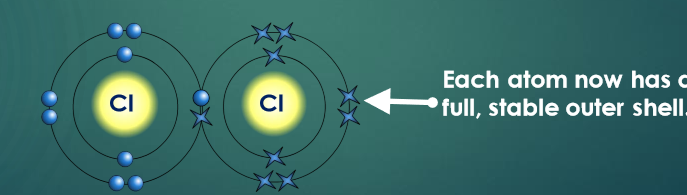
What is a covalent network substance?
A substance with a giant lattice of atoms bonded by strong covalent bonds.
high melting point
hard and non-conductive (e.g. diamond)
What are covalent molecular solids
Small molecules held together by weak intermolecular forces (forces between, not within)
E.g. Hydrogen bonds
low melting points
non-conductive
E.g. bonds between carbon dioxide and/or water molecules
Covalent network vs covalent molecular substances
Feature | Covalent Network | Covalent Molecular |
|---|---|---|
Structure | Giant lattice of atoms bonded by strong covalent bonds | Small, separate molecules held together by weak forces |
Bonding | Strong covalent bonds throughout the whole structure | Strong covalent bonds within molecules, but weak intermolecular forces between them |
Melting & Boiling Points | Very high (due to strong bonds throughout) | Low (due to weak forces between |
How to write ionic formula
Formula of a compound uses chemical symbols and numbers to show the ratio of atoms of each element present
Write down the name for each element
The metal is always written first
Calculate the charge for each type of ion
Balance the number of ions so that the positive and negative charges are balanced and equal zero. This gives the ratio of ions.
Use the ratio to write down the formula of the ionic compound
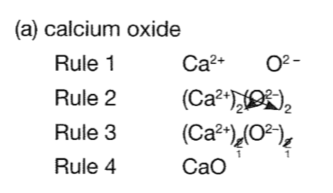
Describe the formation of ionic compounds
Millions and millions of ions are packed together in a regular cubic arrangement, joined by ionic bonds.
Ionic compounds form a giant 3D structure called an ionic lattice
The ionic lattice will continue to build this way until there are no more ions left to add
The structure of the ionic lattice affects the properties of the ionic compound
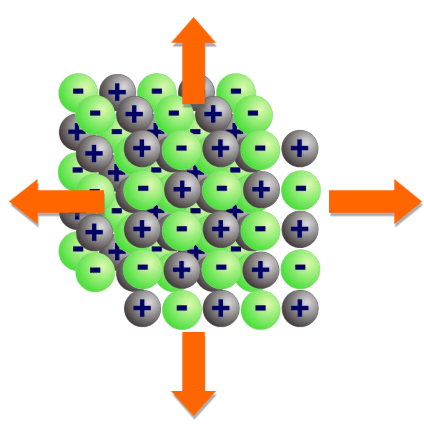
Describe covalent bond properties
Low melting and boiling points
Usually gases or liquids at room temperature
Poor electrical conductivity
Insoluble or slightly soluble in water
Form between non-metal atoms
Share electrons between atoms
How to name covalent substances?
Elements are written in order of left to right on the periodic table
The second element has the suffix “-ide”
Elements are given a prefix to state the number of atoms (except one atom of the first element)
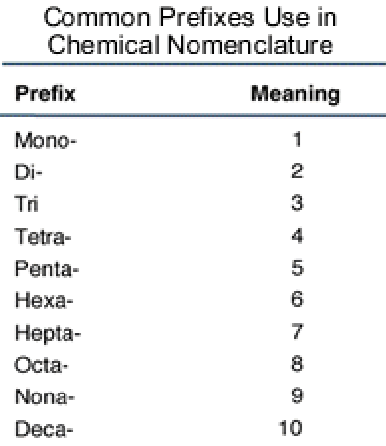
How do you write the formula for a covalent substance?
Use the molecular formula showing the number of atoms of each element in one molecule.
What is the law of conservation of mass?
Mass cannot be created or destroyed in a chemical reaction.
How to name metallic bonds?
Name the metal (cation) first — just the element name.
Name the non-metal (anion) second — use the root of the element + -ide ending.
Examples:
NaCl → Sodium chloride
MgO → Magnesium oxide
CaF₂ → Calcium fluoride
What is a decomposition reaction?
When a single reactant breaks apart to form several products, the reactant is said to decompose.
XY → X + Y
An example is the decomposition of carbonic acid into H2O and CO2.
H2CO3 (aq) → H2O (l) + CO2 (g)
Describe a precipitation reaction
Sometimes two clear solutions are mixed together and go cloudy
This means that an insoluble substance has been created which comes out of solution and forms a solid
This is called a precipitate
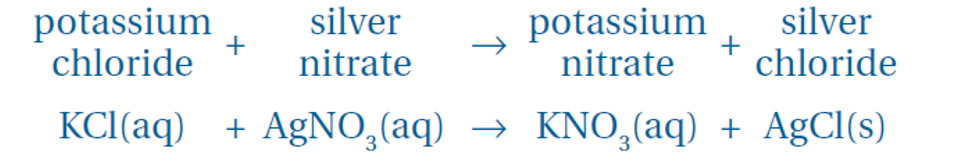
Acid-Metal Reaction
Word: Acid + Metal → Salt + Hydrogen gas
Example: Hydrochloric acid + Zinc → Zinc chloride + Hydrogen gas
Formula: 2HCl + Zn → ZnCl₂ + H₂
How to name salt?
Name the metal (cation) first.
Name the acid’s negative ion (anion) second.
If acid ends with -ic, salt ends with -ate (e.g., sulfuric acid → sulfate).
If acid ends with -ous, salt ends with -ite (e.g., sulfurous acid → sulfite).
If acid is hydrochloric acid, salt ends with -ide (chloride).
Acid-Base (Neutralisation) Reaction
Word: Acid + Base → Salt + Water
Example: Hydrochloric acid + Sodium hydroxide → Sodium chloride + Water
Formula: HCl + NaOH → NaCl + H₂O
Acid-Carbonate Reaction
Word: Acid + Carbonate → Salt + Water + Carbon dioxide
Example: Hydrochloric acid + Calcium carbonate → Calcium chloride + Water + Carbon dioxide
Formula: 2HCl + CaCO₃ → CaCl₂ + H₂O + CO₂
Precipitation Reaction
Word: Solution A + Solution B → Precipitate + Solution C (aqueous)
Example: Silver nitrate + Sodium chloride → Silver chloride (precipitate) + Sodium nitrate
Formula: AgNO₃ (aq) + NaCl (aq) → AgCl (s) + NaNO₃ (aq)
Precipitate should be named like a salt
Metal Displacement Reaction
Word: More reactive metal + Metal salt solution → Salt of more reactive metal + Less reactive metal
Example: Zinc + Copper sulfate → Zinc sulfate + Copper
Formula: Zn + CuSO₄ → ZnSO₄ + Cu
What is a salt?
A chemical compound formed when an acid reacts with a base, metal, or carbonate, where the hydrogen ion (H⁺) from the acid is replaced by a metal or another positive ion.
Write and predict observations for the above reactions.
Reaction Type | Word Equation | Formula Equation | Observations |
|---|---|---|---|
Acid-Base (Neutralisation) | Acid + Base → Salt + Water | HCl + NaOH → NaCl + H₂O | No fizzing, solution may warm, neutral pH |
Acid-Carbonate | Acid + Carbonate → Salt + Water + CO₂ | 2HCl + CaCO₃ → CaCl₂ + H₂O + CO₂ | Fizzing/bubbles (CO₂ gas), solid dissolves |
Acid-Metal | Acid + Metal → Salt + Hydrogen gas | 2HCl + Zn → ZnCl₂ + H₂ | Fizzing/bubbles (H₂ gas), metal dissolves |
Precipitation | Solution A + Solution B → Precipitate + Solution C | AgNO₃ + NaCl → AgCl (s) + NaNO₃ | Solid forms (cloudy/milky), particles settle |
Metal Displacement | More reactive metal + Metal salt → New salt + Metal | Zn + CuSO₄ → ZnSO₄ + Cu | Solid metal forms (colour change), metal dissolves |
What is a lattice?
A regular, repeating 3D arrangement of atoms, ions, or molecules in a solid.
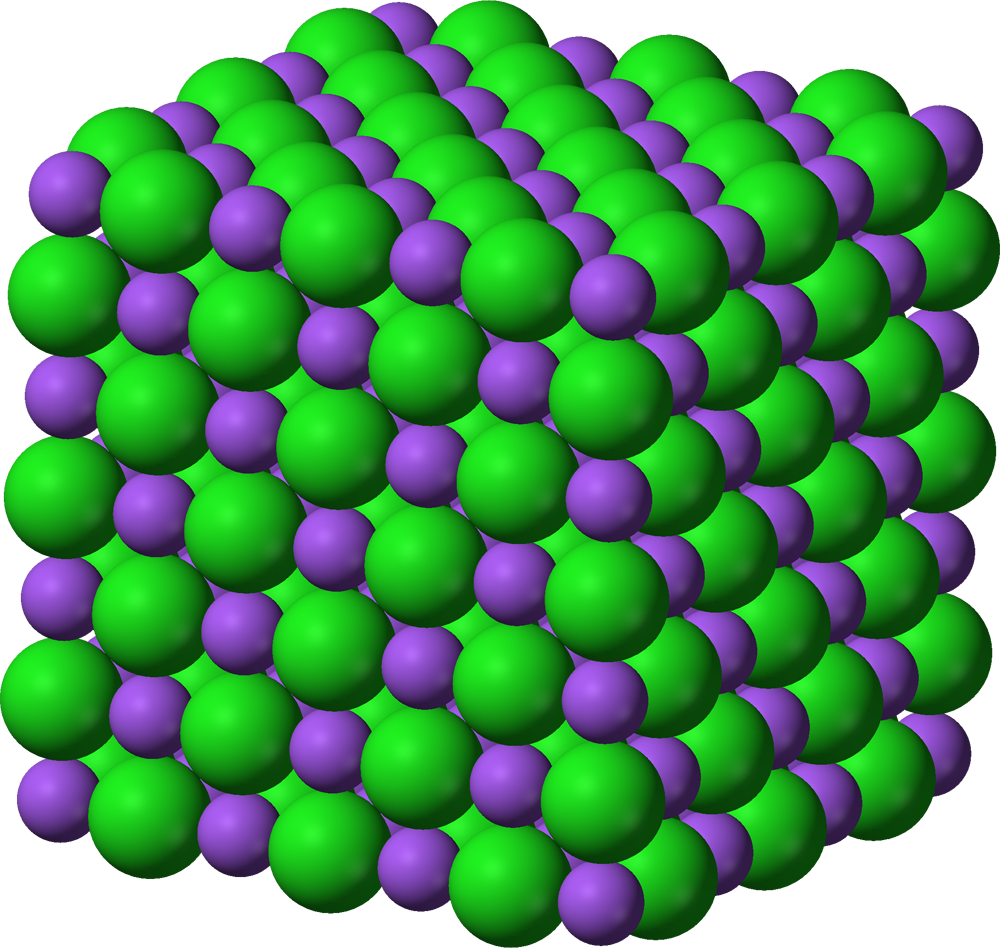
What is an alloy?
An alloy is a mixture of two or more metals, or a metal with another element, combined to make a material with better properties like strength or resistance to rust.
When do you use brackets in ionic bonding formulas?
Use brackets when there is more than one polyatomic ion (a group of atoms) in the compound to show how many of that group are present.
Example:
Calcium nitrate = Ca(NO₃)₂ (two nitrate groups)
What’s the difference between an atom and a molecule?
Atom: One single particle (e.g. H)
Molecule: Two or more atoms joined together (e.g. H₂)
Example:
2H = two separate atoms
H₂ = one molecule of two atoms bonded
Why are metallic bonds malleable and ductile?
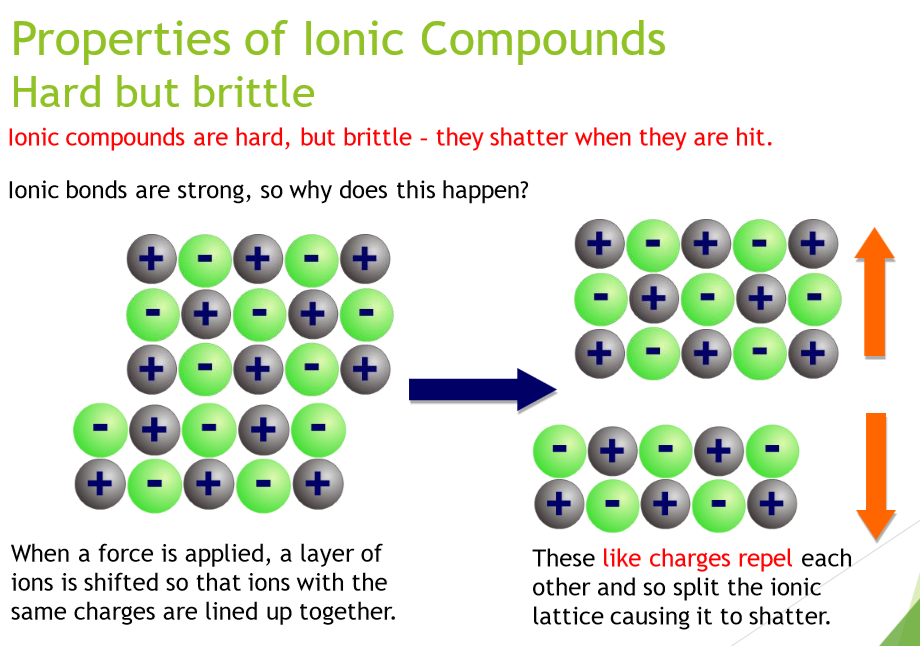
What makes ionic compounds hard but brittle?