OCR A 6.2.3 Polyesters and Polyamides
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
alkenes
-monomers join to form addition polymers
addition polymers
-saturate molecule
-non-polar normally
-unreactive
-don’t degrade well in landfill
condensation polymers
-2 dif monomers with at least 2 func groups react together
-when they react link is made and water is eliminated
-types: polyamides & polyesters
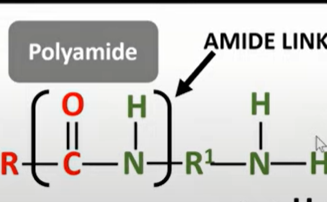
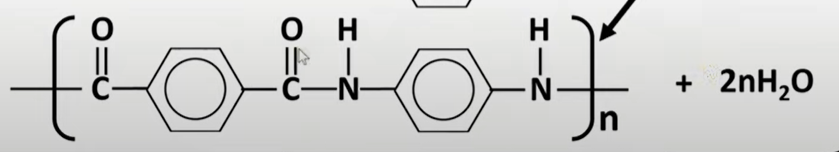
polyamides
-formed by reacting diamines and dicarboxylic acids form amide link
-need func grp to be either side of molecule to form chain
-OH for carboxylic acid and H from amine removed

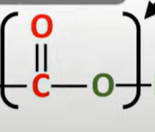
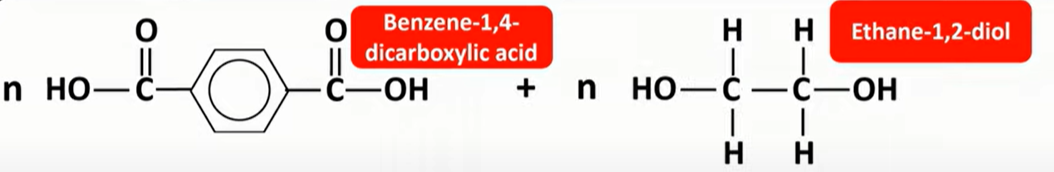
polyesters
-formed by reacting a diol and dicarboxylic acid
-ester links formed
-OH from carboxylic acid
-H from diol
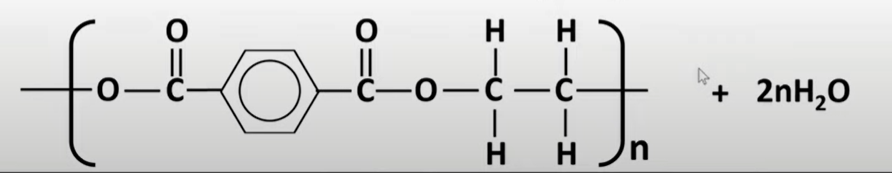
condensation polymer hydrolysis
-split using water to produce orginal monomers
-break in the middle of amide/ester