Atom economy, percentage yield and gas calculations .3
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
25 Terms
what is atom economy
The atom economy of a reaction is a measure of the amount of starting materials that end up as useful products.
what is atom economy important for
It is important for sustainable development and for economic reasons to use reactions with high atom economy.
atom economy =
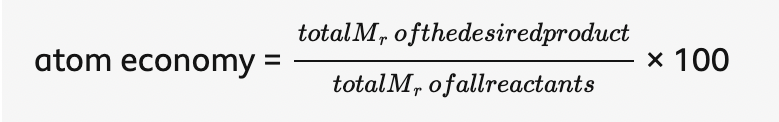
what is the highest possible value of atom economy
100%, when all the reactant atoms end up in the desired product.
If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products.
what is the theoretical yield
The theoretical yield is the maximum possible mass of a product that can be made in a chemical reaction.
how can theoretical yield be calculated
It can be calculated from:
the balanced equation,
the mass and relative formula mass of the limiting reactant, and
the relative formula mass of the product
why is it not always possible to obtain the calculated amount of a given product
Reasons why the mass of product made is less than the maximum theoretical mass include:
the reaction not going to completion, because it is reversible
some of the product may be lost when it is separated from the reaction mixture by filtering, for example
some of the reactants may react in ways different to the expected reaction
percentage yield =
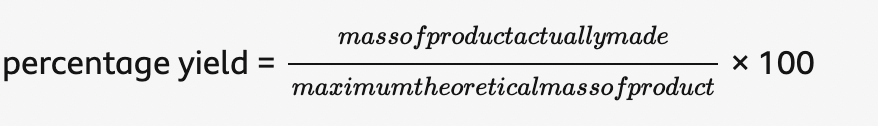
how can the percentage yield vary
100% (no product has been lost)
0% (no product has been made).
what is a reaction pathway
A reaction pathway describes the sequence of reactions needed to produce a desired product.
what does the pathway chosen for a product depends on
percentage yield
atom economy
rate of reaction
equilibrium position
usefulness of by-products
why is the manufacture of ethanol useful and what does the manufacture of ethanol has
The manufacture of ethanol, used as a fuel,
provides a useful example for choosing reaction pathways
which two ways in ethanol manufactured in
Fermentation of plant sugars (C6H12O6(aq) → 2C2H5OH(aq) + 2CO2(g)
Hydration of ethene, obtained from crude oil, using steam (C2H4(g) + H2O(g) → C2H5OH(l)
word equation for Fermentation of plant sugars:
glucose → ethanol + carbon dioxide
which manufacture of ethanol is more effective
Fermentation has a lower percentage yield and rate of reaction (15% - low) than the hydration of ethene (95% - high).
The hydration of ethene has an atom economy of 100%, showing that all the atoms in the reactants form the desired product.
As it also has a higher rate of reaction, the hydration of ethene appears to be the better way to make ethanol.
what type of reaction is the hydration of ethene
reversible reaction
where does the equilibrium position lie in the hydration of ethene
The equilibrium position lies to the left, so only about 5% of the ethene supplied is converted to ethanol
how is the overall yield of the hydration of ethene achieved
The overall yield of 95% is achieved by recirculating unreacted ethene through the reactor.
what is carbon-dioxide a by-product of
Carbon dioxide is a by-product of the fermentation of plant sugars
who can carbon-dioxide be sold to and how does it affect its atom economy
It may be sold to fizzy drinks manufacturers to provide the bubbles in lemonade and cola.
This makes it a desirable product as well, so the atom economy can be increased to 100%.
what does Avogadro's law states about temperature and pressure
Avogadro's law states that when the temperature and pressure stay the same:
equal volumes of different gases contain an equal number of molecules
this means that equal amounts in moles of gases occupy the same volume under the same conditions of temperature and pressure
At a given temperature and pressure what does one mole of gas occupy
the same volume:
what is the molar volume
the molar volume is the volume occupied by one mole of any gas, at room temperature and pressure (same temp + pressure and isnt affected by Mr or Ar of the gas)
amount in mol =
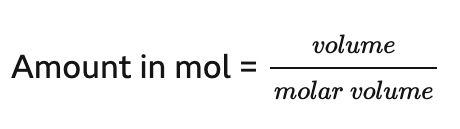
what is the molar volume equal to
molar volume is equal to 24 dm3 (24,000 cm3)