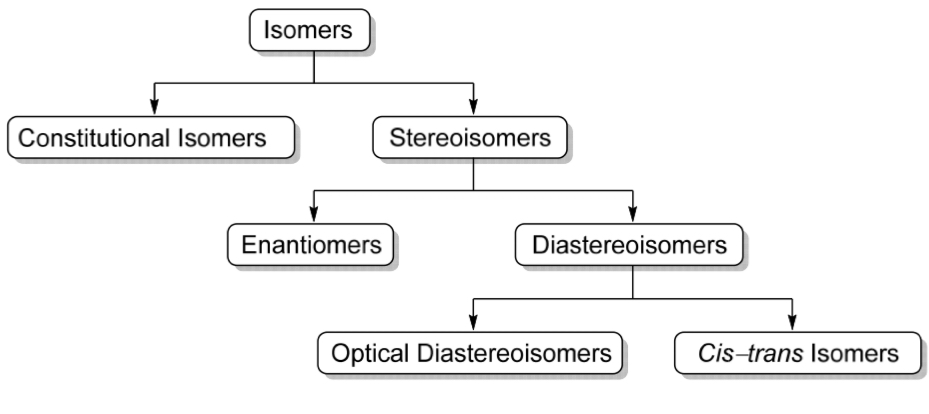
Stereochemistry
1/98
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
99 Terms
Stereoisomers
Molecules with the same connectivity but a different arrangement of atoms in 3 dimensional space (different configurations)
Have the same physical and chemical properties
Constitutional isomers
Have the same molecular formula but different connectivities (order in which the atoms are bonded to each other)
Have very different physical and chemical properties
Why is it difficult to distinguish between some types of stereoisomers in the laboratory?
They often have the same physiochemical properties » same boiling point, melting point, solubility, polarity
Difference between constitutional isomers and stereoisomers
Stereoisomers have different configuration and same connectivity
Constitutional isomers have different connectivity
Which molecules show stereoisomerism?
A Chiral molecule that is non-superimposable on its mirror image
E.g. The 2 stereoisomers of alanine are non superimposable mirror images, therefore these are different compounds
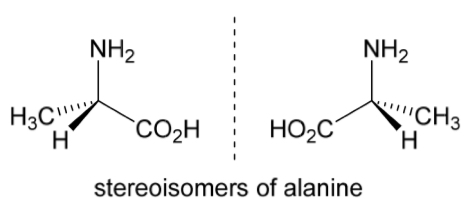
A molecule is chiral if…
It contains a tetrahedral, sp3 hybridised C with FOUR DIFFERENT GROUPS bonded to it
This is called a chirality centre
A molecule is chiral if because it contains a chirality centre
Steps for identifying chirality centres
Discount any C that is not sp3 hybridised
Discount any CH2s and CH3s
How to check if a molecule shows stereoisomerism
Check if the molecule has a chiral C
Draw the mirror image and see if they are non-superimposable
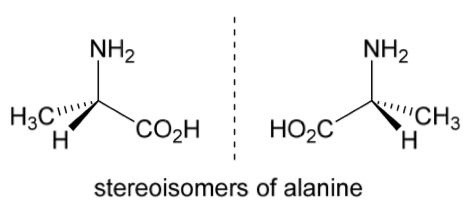
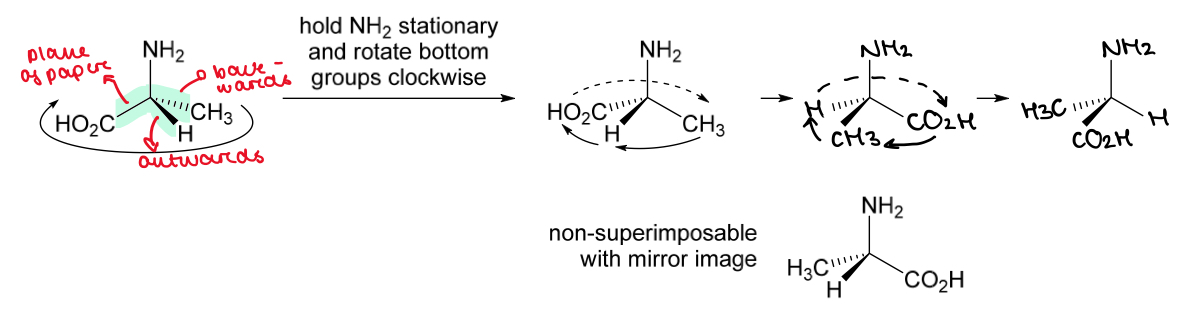
The 2 stereoisomers of alanine are shown.
Rotate the right hand molecule of alanine to demonstrate that it is non-superimposable with the other stereoisomer
No matter how you rotate the structure, it will never be superimposable on its mirror image
So the 2 stereoisomers are 2 different compounds
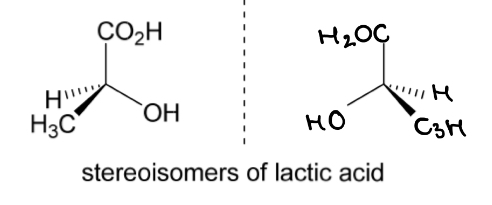
The 2 stereoisomers of lactic acid are shown.
Rotate the left hand molecule of lactic acid to demonstrate that it is non-superimposable with the other stereoisomer
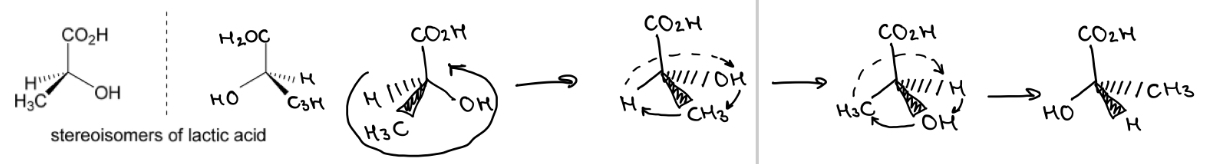
Stereoisomers are…
ENTIRELY DIFFERENT MOLECULES
2 stereoisomers are also called
Enantiomers of one another
Define enantiomers
Non-superimposable mirror images that have OPPOSITE configuration at EVERY chirality centre
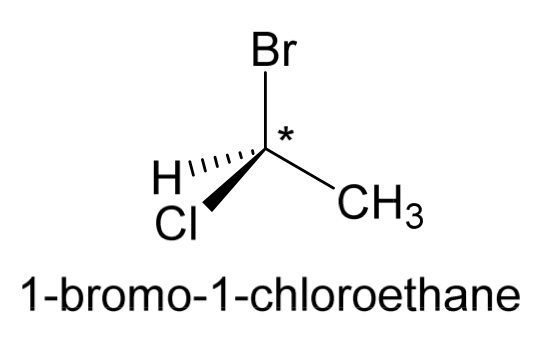
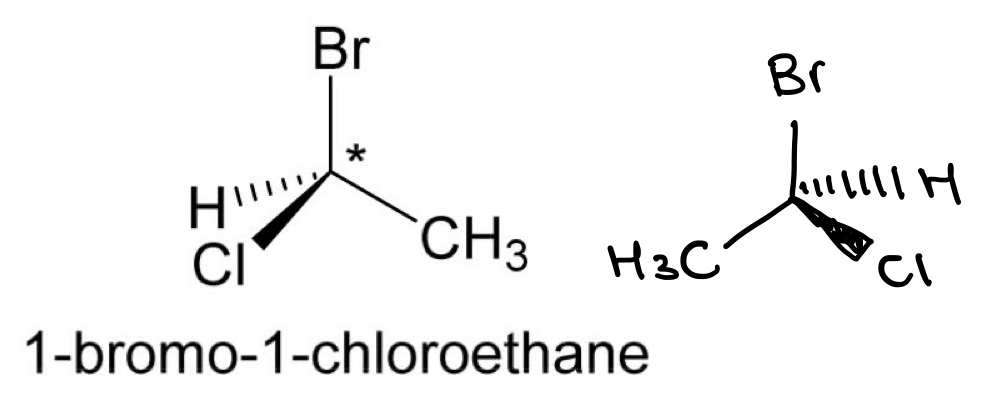
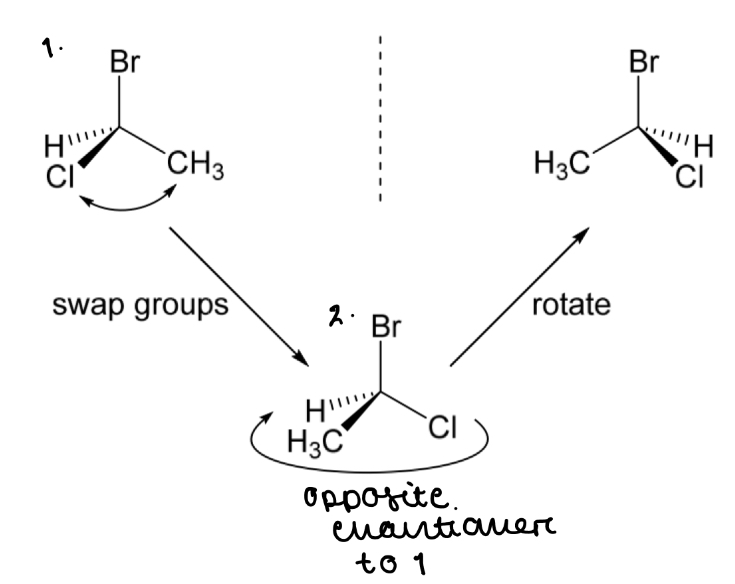
Draw the opposite enantiomer for 1-bromo-1-chloroethane
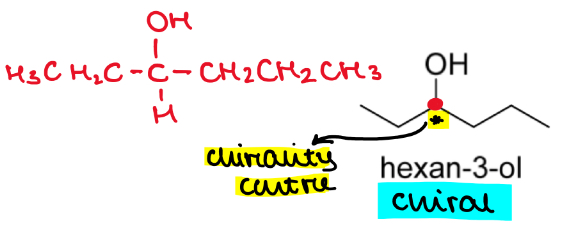
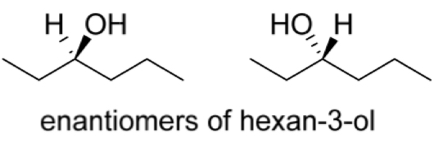
Is hexan-3-ol a chiral molecule?
If so draw an asterisk at the chiral/stereogenic centre
Yes
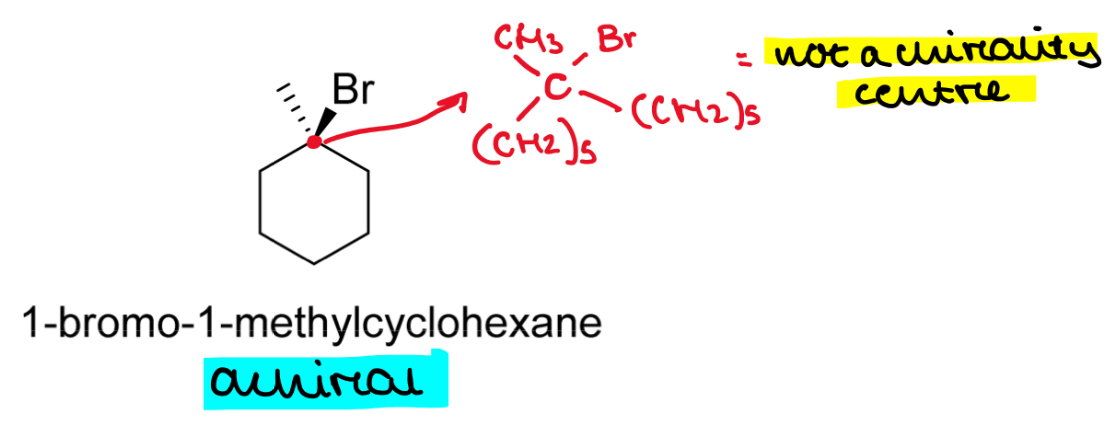
Is 1-bromo-1-methylcyclohexane a chiral molecule?
If so draw an asterisk at the chiral/stereogenic centre
Ignore the CH2 carbons
That just leaves C1, which is bonded to Br and CH3
If we go around the ring clockwise, we have CH2CH2CH2CH2CH2
And if we go anticlockwise, we also have CH2CH2CH2CH2CH2 » molecule is symmetrical
So C1 is not a chirality centre and the molecule is achiral
If a molecule has a plane of symmetry…
No chirality centres
The molecule is achiral
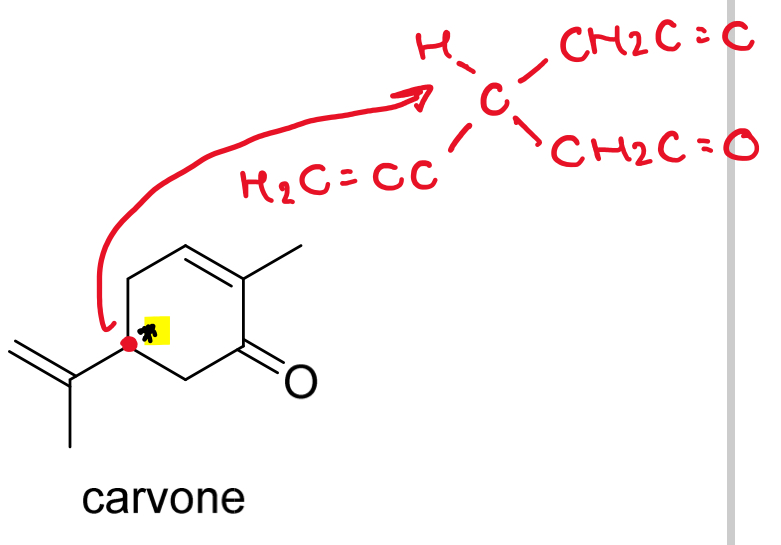
Identify any stereogenic centres in carvone and mark them with asterisks
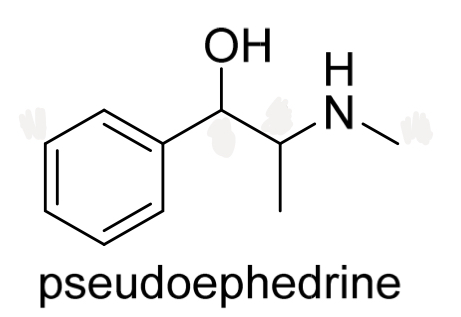
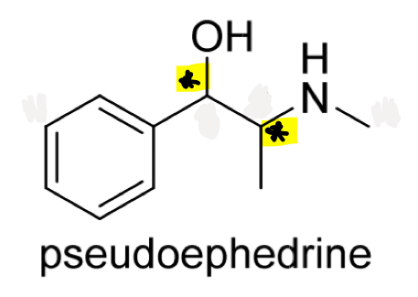
Identify the chirality centres in pseudoephedrine
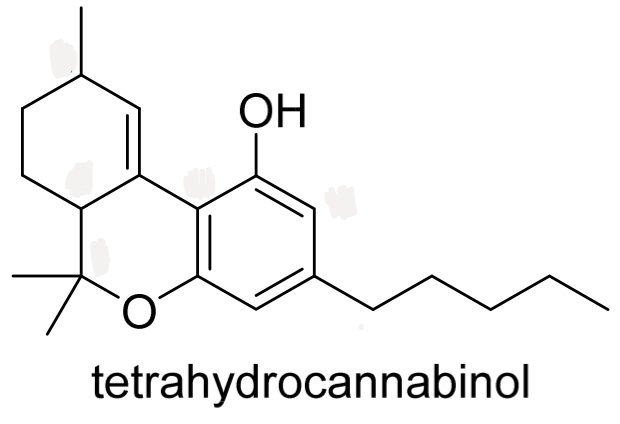
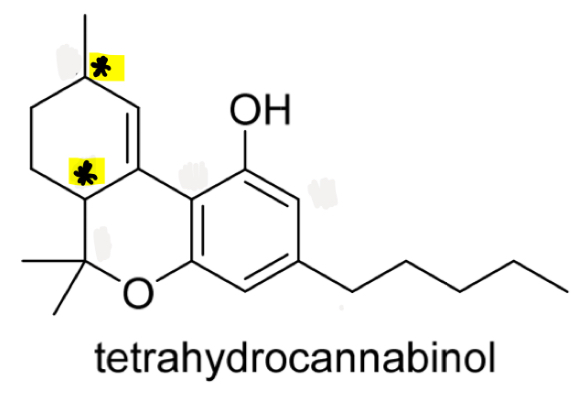
Identify the chirality centres in tetrahydrocannabinol
When is a chiral molecule stereospecific?
Why is it important to be stereospecific when drawing chiral molecules
When it shows how the 4 groups bonded to the stereogenic C atom are arranged in three dimensions
Knowing how the bonds are arranged in a chiral molecule allows us to distinguish between enantiomers
How do we draw a chiral molecule stereospecifically?
Must draw the stereogenic C as tetrahedral
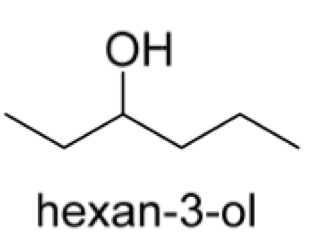
Draw the stereospecific structure of both enantiomers of hexan-3-ol from its bond-line formula shown
2 ways of drawing enantiomers
Reflecting the structure to form the mirror image
By swapping 2 groups then rotating the molecule
Draw the 2 enantiomers of bromo-chloro-ethane using the 2 different methods:
Mirror image
Swapping the substituents and rotating
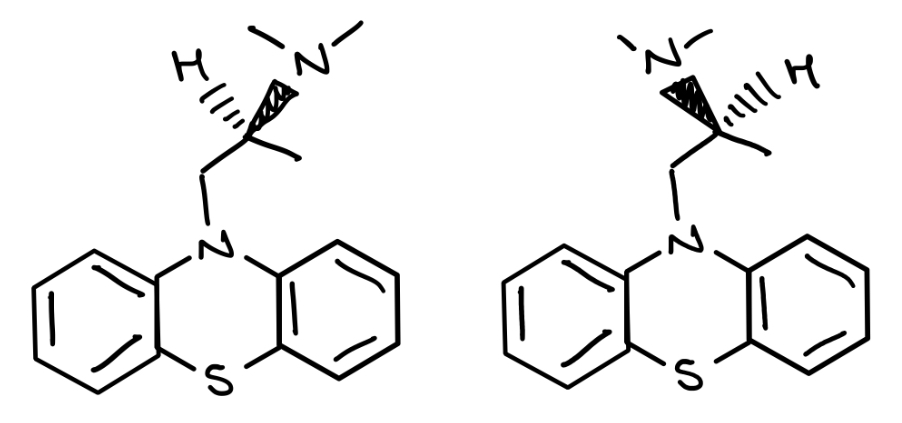
Draw the individual enantiomers of promethiazide in any suitable stereospecific form
Why do we need a method of describing in words the configuration of different stereoisomers?
The systematic name of a compound describes connectivity, but enantiomers have the SAME connectivity
And we cannot use the same name to describe configuration for each enantiomer because they are DIFFERENT COMPOUNDS
What system do we use to describe the configuration of a chirality centre of 2 enantiomers
The Cahn-Ingold-Prelog
AKA
R-S convention
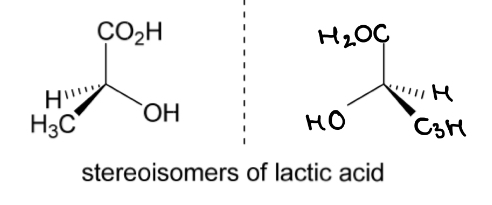
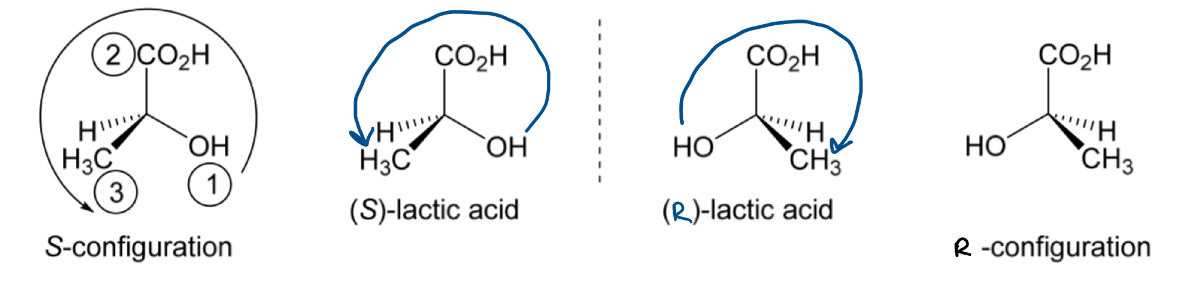
The 2 enantiomers of lactic acid are shown.
Which one is (S)-lactic acid and which is (R)-lactic acid?
Identify the 4 different substituents bonded to the chirality centre: CO2H, OH, CH3, H
Rank the substitutents in order of decreasing priority by ranking them according to atomic number. If the directly bonded atoms are the same move along to the next atom: OH > CO2H > CH3 > H
Draw the molecule with the lowest priority group behind the plane of the paper
If the lowest priority group is not already at the back: pick one of the bonds in the plane of the paper and keep that group fixed (not the lowest priority group as this is the one you want to move), rotate the other groups until the lowest priority one is at the back
Draw an arrow starting from the 1st to 2nd to 3rd highest priority substituent
If the arrow is anticlockwise = S configuration
If the arrow is clockwise = R configuration
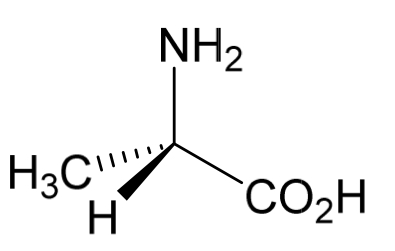
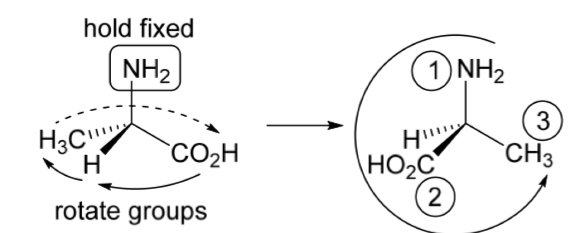
Determine the configuration of the stereoisomer of alanine shown
Anti-clockwise arrow
So (S)-alanine
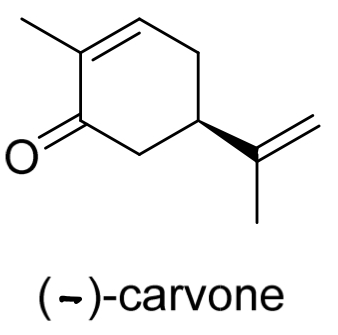
Determine the configuration of (-)-carvone
(R)-carvone
IF A SUBSTITUENT HAS A C=C IT HAS HIGHER PRIORITY THAN A C-C
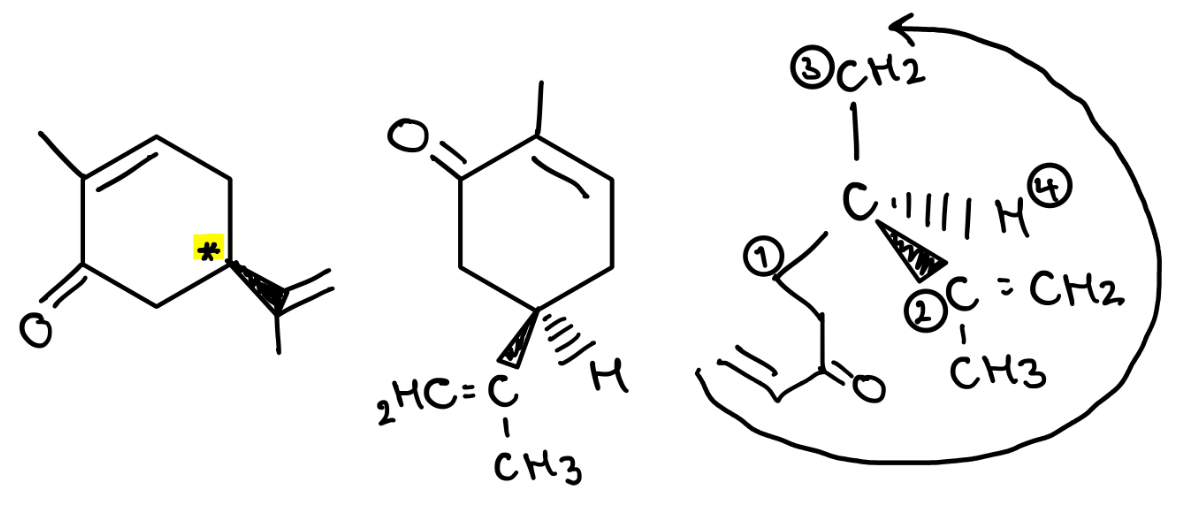
How to draw a particular enantiomer when given the configuration (R or S)
Identify chirality centre
Rank the constituents
Place the lowest priority substituent in the back
Order the other substituents either clockwise (if R) or anticlockwise (if S) in order of priority (1, 2, 3, 4)
If asked to determine absolute configuration…
This is whether it is R or S
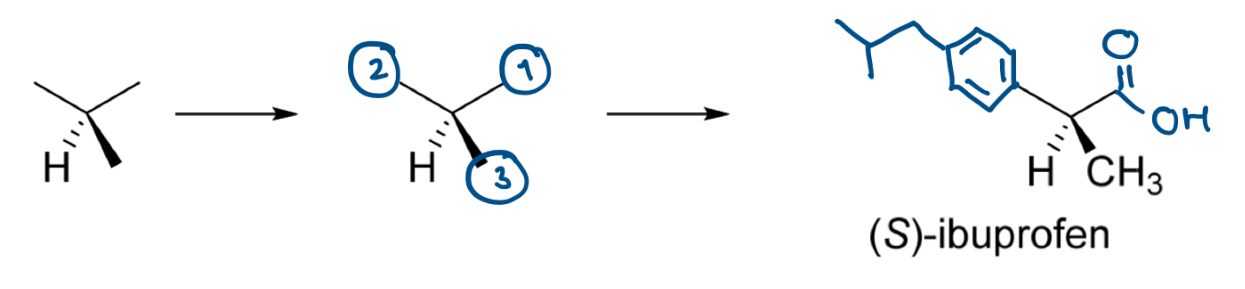
Draw the biologically active S enantiomer of ibuprofen
Identify chiral C
Order of priority is -COOH > aromatic ring > CH3 > H
Draw 3D structure with H at the back
Order the other 3 substituents anticlockwise (can be in the order that makes it the most easy to draw, just has to be anticlockwise)
Physical properties of enantiomers
Same physical properties
The molecules of each enantiomer pack together in the same way with the same intermolecular forces » so the density, melting point and boiling point for the S enantiomer will be the same as the R enantiomer
The overall polarities of the 2 enantiomers are the same = same solubility
What is the only physical property enantiomers can be distinguished by?
Their interaction with plane polarised light
What happens if you pass plane polarised light through a solution of a chiral substance?
ONLY chiral substances are optically active
The substance will cause plane polarised light to be rotate through a certain angle (α)
The angle by which the polarised light is rotated can be measured using another polarising filter on the other side of the sample which is turned until the light passes through it again » called a polarimeter
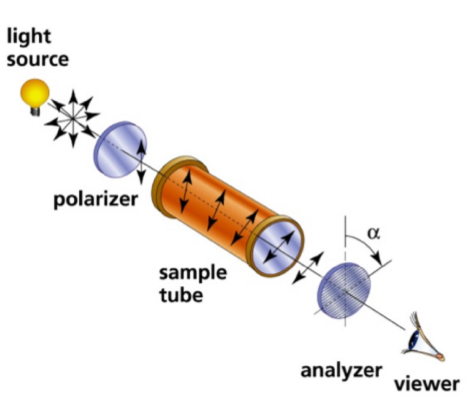
How can we classify chiral molecules based on how they rotate plane polarised light?
If a substance causes plane-polarised light to rotate clockwise, α has a positive value
» substance is dextrorotatory
» (+)-name of substance e.g. (+)-carvone
If plane polarised light rotated anticlockwise, α has a negative value
» substance is levorotatory
» (-)-name of substance e.g. (-)-carvone
What does the angle of rotation (α) depend on?
Concentration (% or g/100mL)
Path length (dm)
How to calculate the angle of rotation
[α] = 100α /concentration (% or g/100mL) x path length (dm)
[α] = 100α / c x l
Measured at 25oC, using light of wavelength 589.6 nm
What is the relationship between the rotation of plane polarised light pair of enantiomers?
Equal in magnitude but opposite in direction
The [α] value for (R)-lactic acid is -3.33o. What is the [α] value for (S)-lactic acid?
+3.33o
Chemical properties of enantiomers
Same chemical properties
Same polarity
Same acidity » have same pKa
Same reactivity
However, react differently with other chiral molecules
Pharmacological activity of enantiomers (how do they act in the body)
Target molecules in the body e.g. receptors and enzymes are usually proteins
Proteins are chiral as made up of chiral amino acids
They have specific binding sites where only drugs with a complementary shape can bind
For a chiral drug, only one enantiomer will have the complementary shape to bind to the binding site
So one enantiomer is active and the other is inactive
Why is it not good practise to administer an inactive enantiomer to a patient?
Sometimes the opposite enantiomer is toxic e.g. thalidomide
Relative configuration
What is the standard to which other compounds are compared to?
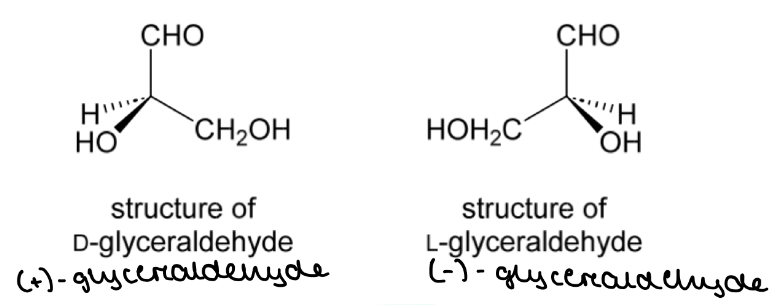
Glyceraldehyde
The configuration of substituents in (+)-glyceraldehyde is called the ‘D’ configuration
The configuration of substituents in (-)-glyceraldehyde is called the ‘L’ configuration
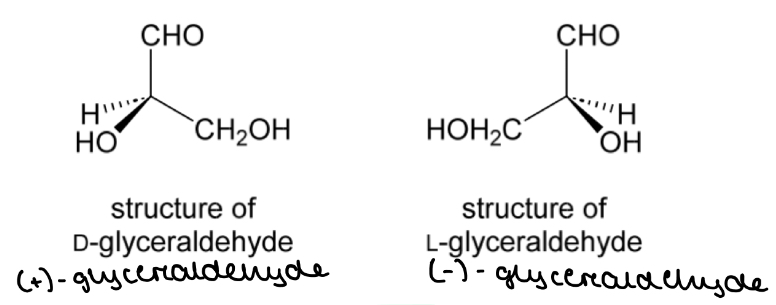
Relative configuration
How is glyceraldehyde used as a standard to describe other enantiomers?
Compounds that are chemically similar to D-glyceraldehyde = D configuration
Compounds that are chemically similar to L-glyceryladehyde = L configuration
All amino acids found in proteins are __ configuration
L configuration
All the carbohydrates found in nature are __ configuration
D configuration
Compounds with only 1 chirality centre only have…
2 stereoisomers
The R enantiomer and the S enantiomer (which are non-superimposable mirror images of each other)
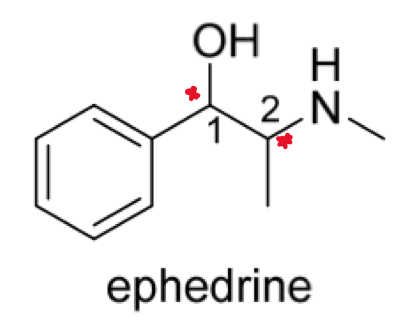
How do we stereospecifically represent compounds that have MORE THAN ONE chirality centre? E.g. ephedrine
The structure contains TWO chirality centres (C1 and C2)
C1 could be R or S configuration and C2 could also be R or S configuration
This gives a total of 4 possible stereoisomers:
(1R,2R)-, (1R,2S)-, (1S,2S)-, (1S,2R)-
Start with the stereoisomer that has R configuration at each chirality centre » (1R,2R)-ephedrine
If we draw the mirror image of this » (1S,2S)-ephedrine
If we change the configuration of C1 only, by swapping the 2 substituents (the order of substituents now goes anticlockwise) » (1S,2R)-ephedrine
If we draw the mirror image of this » (1R,2S)-ephedrine
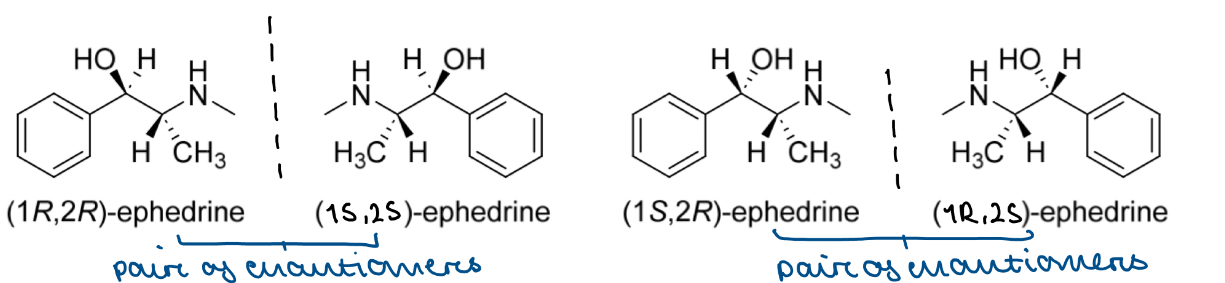
What is the relationship between all the stereoisomers of a compound with MORE THAN ONE chirality centre
E.g. for a compound with TWO chirality centres
(1R,2R) and (1S,2S) are enantiomers
(1S,2R) and (1R,2S) are enantiomers
So the 4 stereoisomers exist as 2 pairs of enantiomers
The stereoisomers that are not enantiomers of each other [e.g. (1R,2R) and (1R,2S), (1S,2S) and (1S,2R) etc] are diastereoisomers of each other
These are stereoisomers that differ in configuration at one or more chirality centre but are NOT mirror images of each other
Draw any stereoisomer of 2-bromo-3-chlorobutane in a suitable stereospecific form and determine its absolute configuration. Draw its enantiomer and any diastereoisomer and write down their absolute configurations
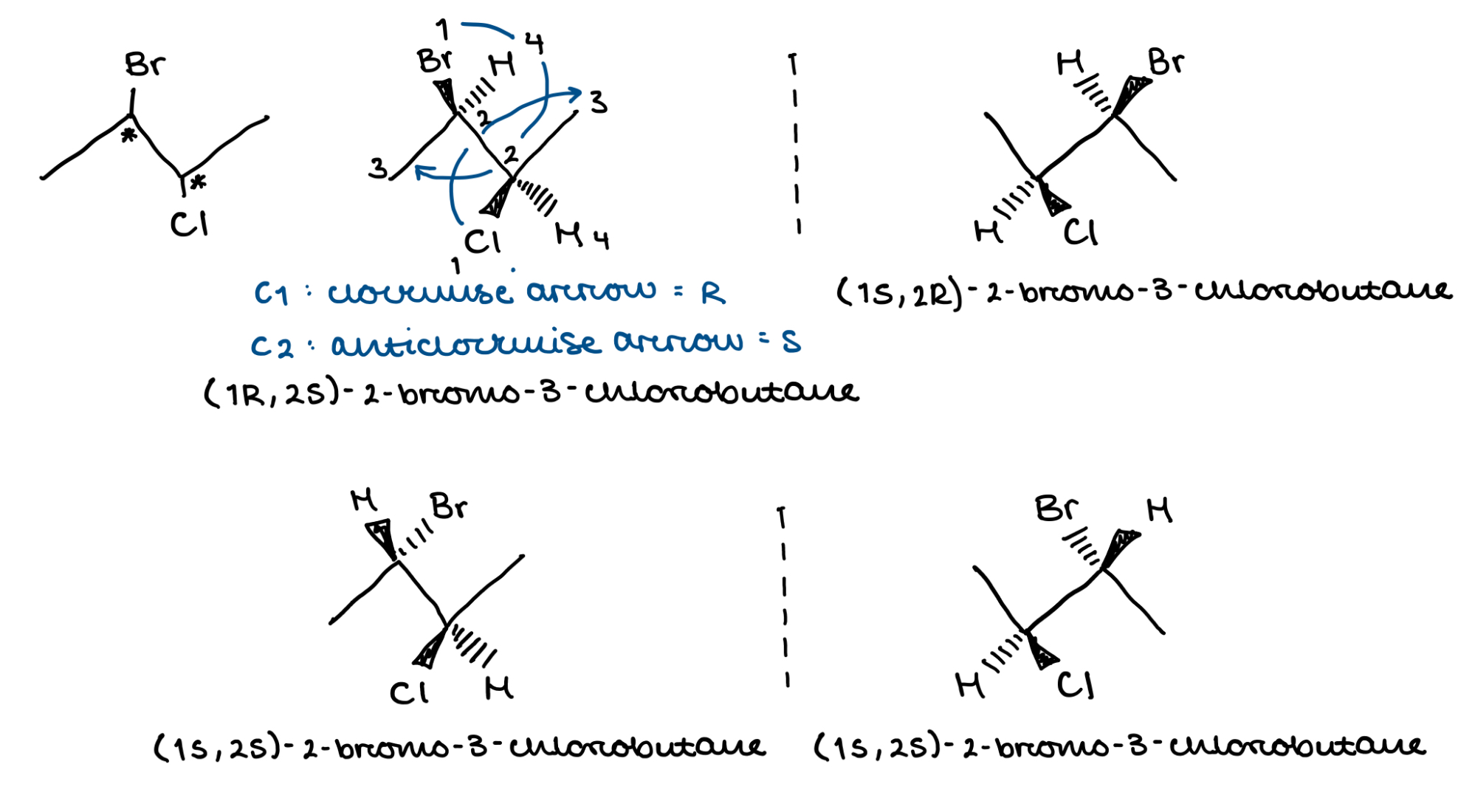
If you’re confused about how swapping the 2 substituents on C1 changes the configuration from R to S even though it seems like the substituents still go in a clockwise order:
If you hold a group in the plane of the paper fixed and you rotate the substituents so the H is at the back, you’ll find IT DOES GO IN AN ANTICLOCKWISE ORDER YIPPEE !!!!!
Diastereoisomers
Stereoisomers that differ in configuration at one or more stereogenic centres but are NOT mirror images of each other
Physical properties of diastereoisomers
e.g. (1R,2R) and (1R,2S)
e.g. (1S,2S) and (1S,2R)
e.g. (1R,2R) and (1S,2R)
e.g. (1S,2S) and (1R,2S)
Diastereoisomers are completely different compounds
Different melting points, boiling points, solubilities and reactivities
Diastereoisomers are optically active and differ in the direction AND angle they rotate plane polarised light
If a compound has TWO chirality centres…
Maximum of 4 possible stereoisomers
RR, SS, RS, SR
Formula for calculating maximum number of stereoisomers
2n
n = number of chirality centres
WHEN WILL A COMPOUND NOT BE CHIRAL
If it has a plane of symmetry
These are called meso compounds
How many stereoisomers are possible for a molecule with 2 stereogenic centres?
23 = 8
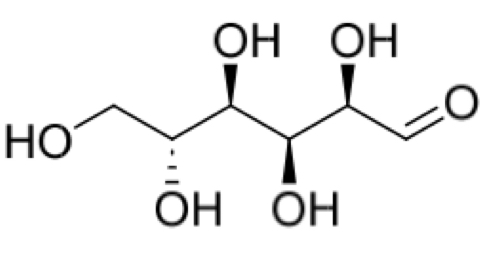
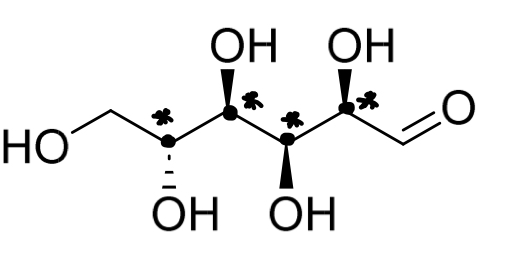
The open chain form of glucose is shown. What is the maximum total number of stereoisomers possible for this sugar?
4 chirality centres
24 = 16
Cis-trans isomerism
Type of stereoisomer
Same connectivity, but different arrangement of their groups in space (different configuration)
Why do cis-trans isomers have different configurations?
Because there is no free rotation about the carbon-carbon double bond
So it is not possible to convert one isomer into another without breaking and reforming bonds
Why are cis-trans isomers technically diastereoisomers?
They differ in configuration but are NOT mirror images
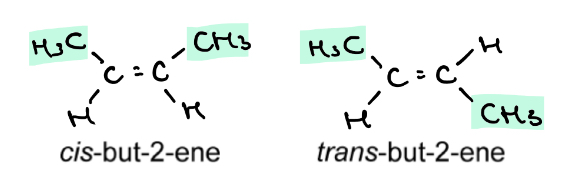
Why is cis-but-2-ene and trans-but-2-ene not optically active?
They have symmetry and so are superimposable on their own mirror images
So these molecules are achiral and do not rotate plane polarised light
Why are cis and trans molecules always achiral
We only use the prefixes cis and trans when each C in C=C is bonded to a H and one other group
This means they will always be symmetrical and non-superimposable on their mirror image
Properties of cis-trans isomers
Cis-trans isomers are diastereoisomers
So have DIFFERENT physical, chemical and pharmacological properties
Differences in the properties of trans-fatty acids and cis-fatty acids
Trans-fatty acids:
» have linear chains which can pack together more easily = higher melting point
» more stable than cis-fatty acids as the substituents are further away from each other in space so there is repulsion
Cis-fatty acids:
» the cis double bond results in a bend in the chain, causing less efficient packing = lower melting point
When is the only time you use cis and trans?
When each C in the double bond has a HYDROGEN and one other substituent
SAME SIDE HIGHEST PRIORITY SUBSTITUENTS =
CIS
OPPOSITE SIDE HIGHEST PRIORITY SUBSTITUENTS =
TRANS
What do we use if each C in the C=C is not bonded to a H?
E and Z
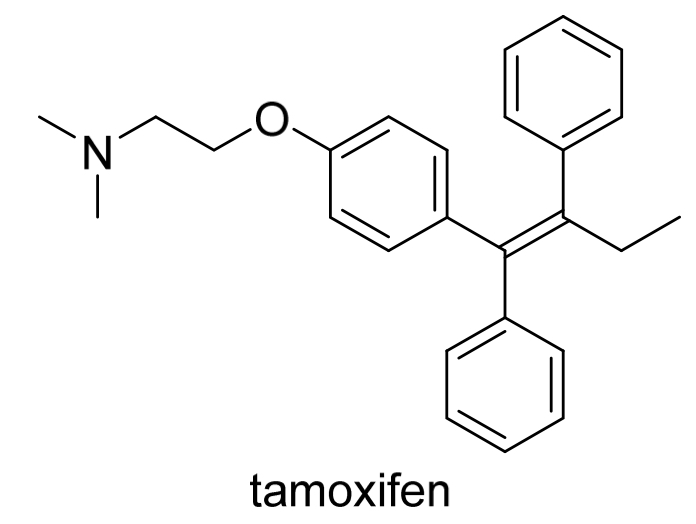
The oestrogen receptor antagonist tamoxifen is used in breast cancer. Only the stereoisomer shown is pharmacalogically active. Determine whether it has E or Z configuration
Identify where the C=C is and whether each C is bonded to 2 different groups
Find the highest priority group for each C in C=C
Both on the same side
(Z)-tamoxifen
Categorise all the different types of isomers
When a reaction with a chiral molecule takes place, what does the configuration of the molecule depend on?
Depends on the part of the molecule at which the reaction takes place
If the reaction is away from the chirality centre = the configuration of the product will be the same as the substrate (retention of configuration)
If the reaction takes place at the chirality centre = different configuration in the product to the substrate
Chiral compounds that undergo SN2 nucleophilic substitution will always result in…
Inversion of configuration
This is because the nucleophile must attach from the side of the molecule opposite from the side which the leaving group departs
E.g. configuration will change from R to S
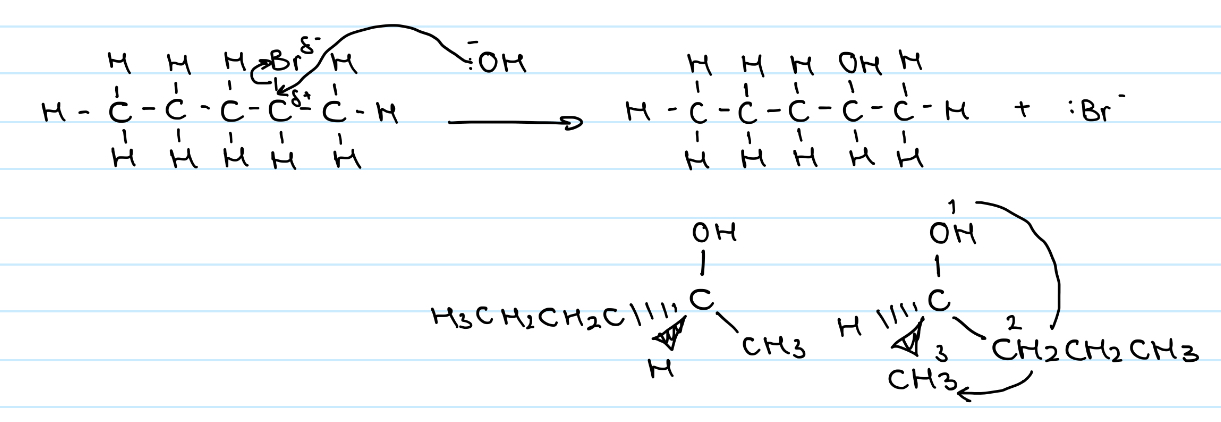
Draw the SN2 nucleophilic substitution mechanism for (R)-2-bromobutane reaction with hydroxide
One-step reaction
Attack of the nucleophile occurs simultaneously with the loss of the leaving group
Inversion of configuration means (R)-2-brompropane is converted to (S)-butan-2-ol
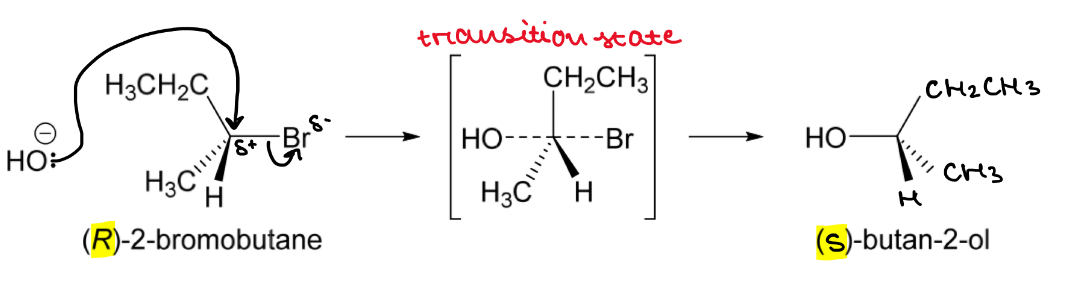
2-bromobutane ALWAYS UNDERGOES
SN2 NUCLEOPHILIC SUBSTITUTION
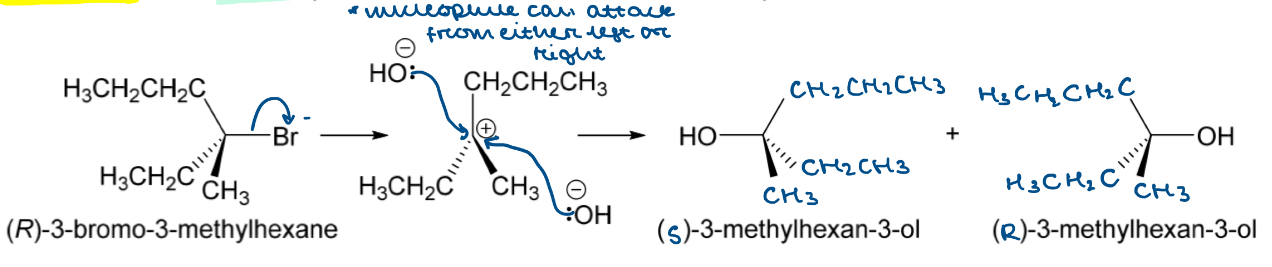
Draw the nucleophilic substitution mechanism for the reaction between (R)-3-bromo-3-methylhexane and hydroxide
Tertiary alkyl halide = SN1
Two-step process
Leaving group leaves during RDS forming carbocation intermediate
Nucleophile attacks the +ve C
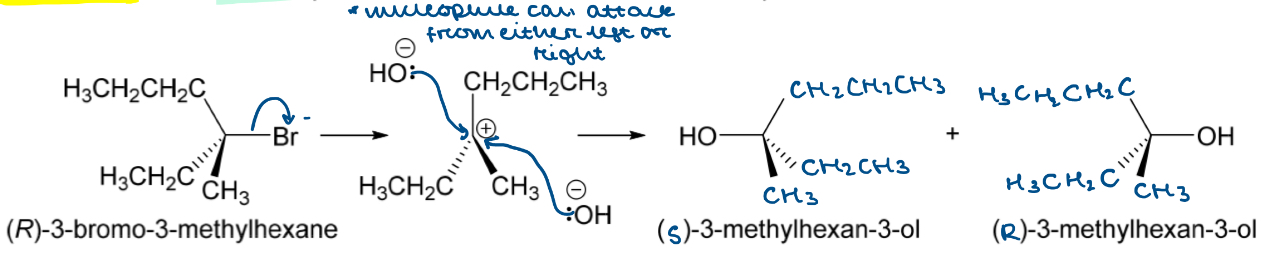
What is racemisation?
The process by which a single stereoisomer is converted into a 1:1 mixture of enantiomers
How can an SN1 nucleophilic substitution result in a racemic mixture?
The nucleophile can attack the +ve carbocation from either the right or the left
There is an equal chance of either happening so an equal amount of each enantiomer is formed
This forms a racemic mixture
Why do racemic mixtures show no optical activity
Mixture contains an equal concentration of the 2 enantiomers
The clockwise rotation of the dextrorotatory enantiomer and the anticlockwise rotation og the levorotatory enantiomer cancel each other out
What is the process where 2 enantiomers are separated from a racemic mixture called?
Resolution of the enantiomers
Describe what it means by chemical reactions in the body being stereoselective
Why are they stereoselective?
They only work on one stereoisomer and produce products that are single stereoisomers
This is because chemical reactions are only catalysed by enzymes which have a specific active site so only a single stereoisomer can bind
Consequences of chemical reactions in the body being stereoselective
Molecules produced by biosynthesis are usually single stereoisomers e.g. the only isomer of lactic acid produced by anaerobic respiration is (S)-lactic acid
Often one enantiomer will be a substrate for an enzyme and the other will not » if a drug is administered as a racemic mixture, the enantiomers will have different activity and will be metabolised differently
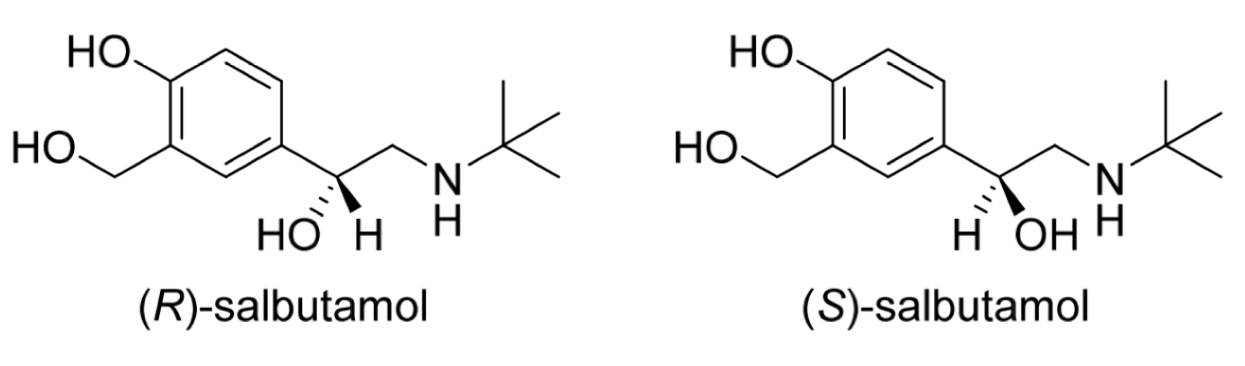
Salbutamol
How many chirality centres?
How is the drug administered?
Characteristics of each enantiomer
1 chirality centre
Salbutamol is administered as a racemic mixture
Only the R enantiomer is biologically active and is metabolised more quickly
The S enantiomer can cause inflammation
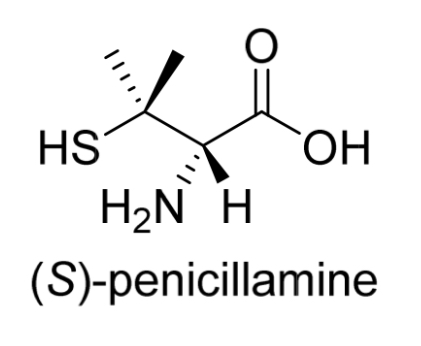
Penicillamine
How many chirality centres?
How is the drug administered?
Amino acid that contains 1 chirality centre
Administered as a single enantiomer, (S)-penicillamine, as the R enantiomer is highly toxic
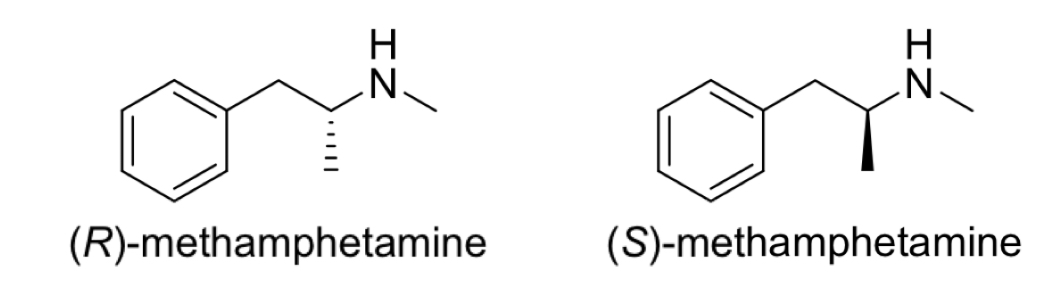
Methamphetamine
How many chirality centres?
Characteristics of each enantiomer
1 chirality centre
The R enantiomer is used in OTC nasal decongestant remedies
The S enantiomer is crystal meth
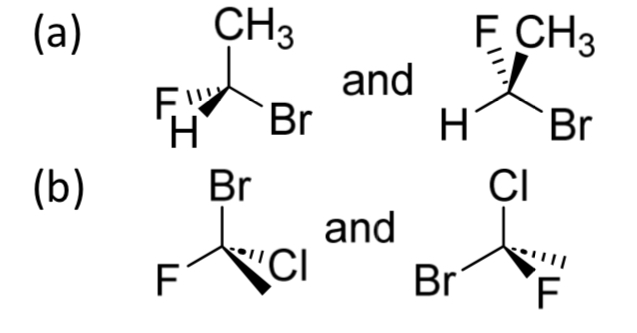
EQ:
Determine if the following pairs of molecules are identical or enantiomer of one another
A)
Br is in the same place in both
All the rest of the groups are in the same order
So the 2 molecules are identical
OR
If you rotate the molecules to put the lowest priority substituent at the back
Both molecules will have S configuration
So they are identical
B)
Rotate molecules to put lowest priority substituent at the back
One molecule is S configuration and the other is R
So they are enantiomers
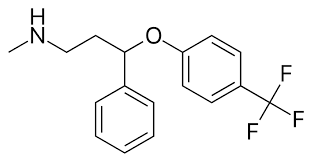
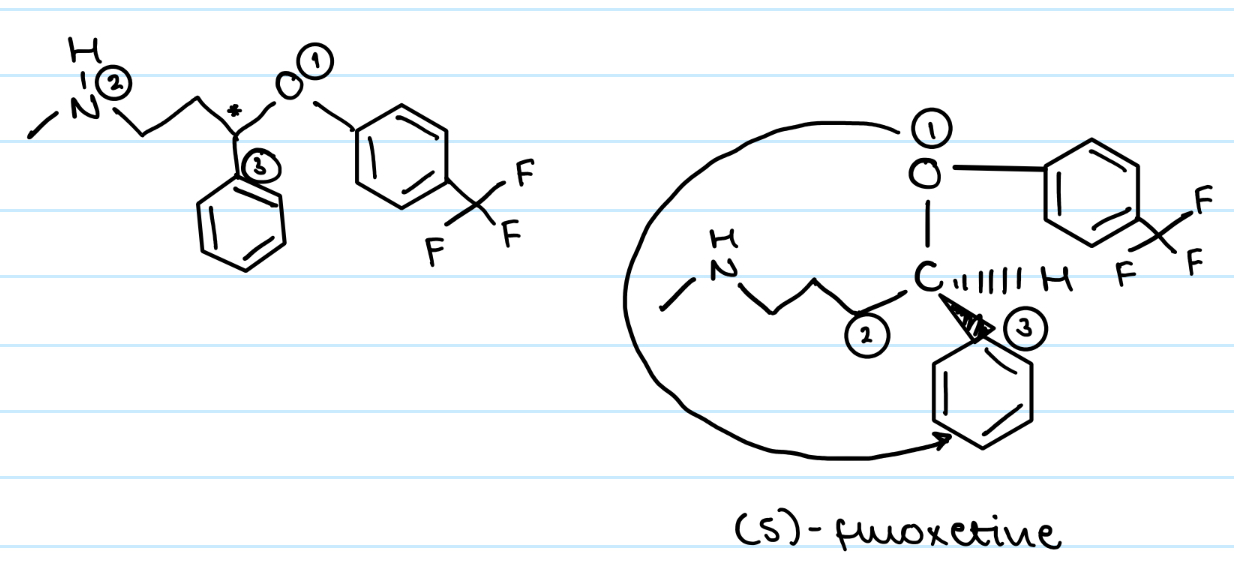
EQ:
Draw the biologically active S enantiomer of fluoxetine.
How will the physical and chemical properties of (S)-fluoxetine compare to those of (R)-fluoxetine
Identical physiochemical properties
Except they rotate plane polarised light in opposite directions and react differently with other chiral molecules
EQ:
250mg of (S)-ibuprofen was dissolved in methanol to produce 10mL of solution. When this solution was analysed at 25oC in a 20cm path length polarimeter, the observed optical rotation, a, was measured at +2.7o. Calculate the value of [a]25 for (S)-ibuprofen.
Predict the value of [a]25 for (R)-ibuprofen and a racemic mixture of ibuprofen.
Concentration of (S)-ibuprofen: 250mg = 0.25g in 10mL = 2.5g/100mL
Path length = 20/10 = 2dm
[a]25 (S)-ibuprofen = 100a/cl = 100 × 2.7 / 2.5 × 2 = +54o
[a]25 (R)-ibuprofen = -54o
[a]25 racemic mixture = 0o
EQ:
Draw the mechanism of the SN2 reaction between (S)-2-bromopentane and sodium hydroxide.
Give the name, including the absolute configuration, of the product that is formed
R-pentan-2-ol
EQ:
Advantages and disadvantages of manufacturing and formulating a drug as a single active enantiomer
+ inactive enantiomer may contribute to unwanted effects
- synthesis and isolation of single enantiomer may be difficult and costly
EQ:
Do diastereoisomers always rotate the plane of polarised light in opposite directions?
NO
When there is an aromatic ring with 1 heteroatom in it
None of the Cs are chiral !!!
IF A QUESTION ASKS WHAT IS THE MAXIMUM NUMBER OF STEREOISOMERS..
CHECK FOR CHIRALITY CENTRES FOR RS ENANTIOMERS
CHECK FOR C=C DOUBLE BONDS FOR EZ IZOMERS
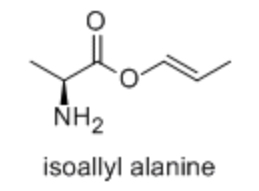
EQ:
What is the maximum number of stereoisomers possible for isoallyl alanine?
4
Whenever deciding whether a molecule is R or S configuration..
ALWAYS DRAW THE DASH AND WEDGE ON THE LEFT SIDE
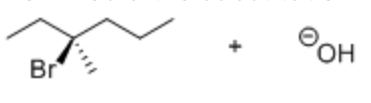
EQ:
How would the substitution reaction shown affect the configuration of the substrate?
Tertiary alkyl halide
So undergoes SN1
Racemisation