Chapter 14: Mixtures and Solutions Overview
1/147
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
148 Terms
Solution
Homogeneous mixtures of two or more pure substances.
Solute
The substance that is dissolved in a solution.
Solvent
The substance in which the solute is dissolved.
Colloid
A mixture where larger particles are dispersed but do not settle out.
Suspension
A temporary heterogeneous mixture with very large solute particles that can be seen with the naked eye.
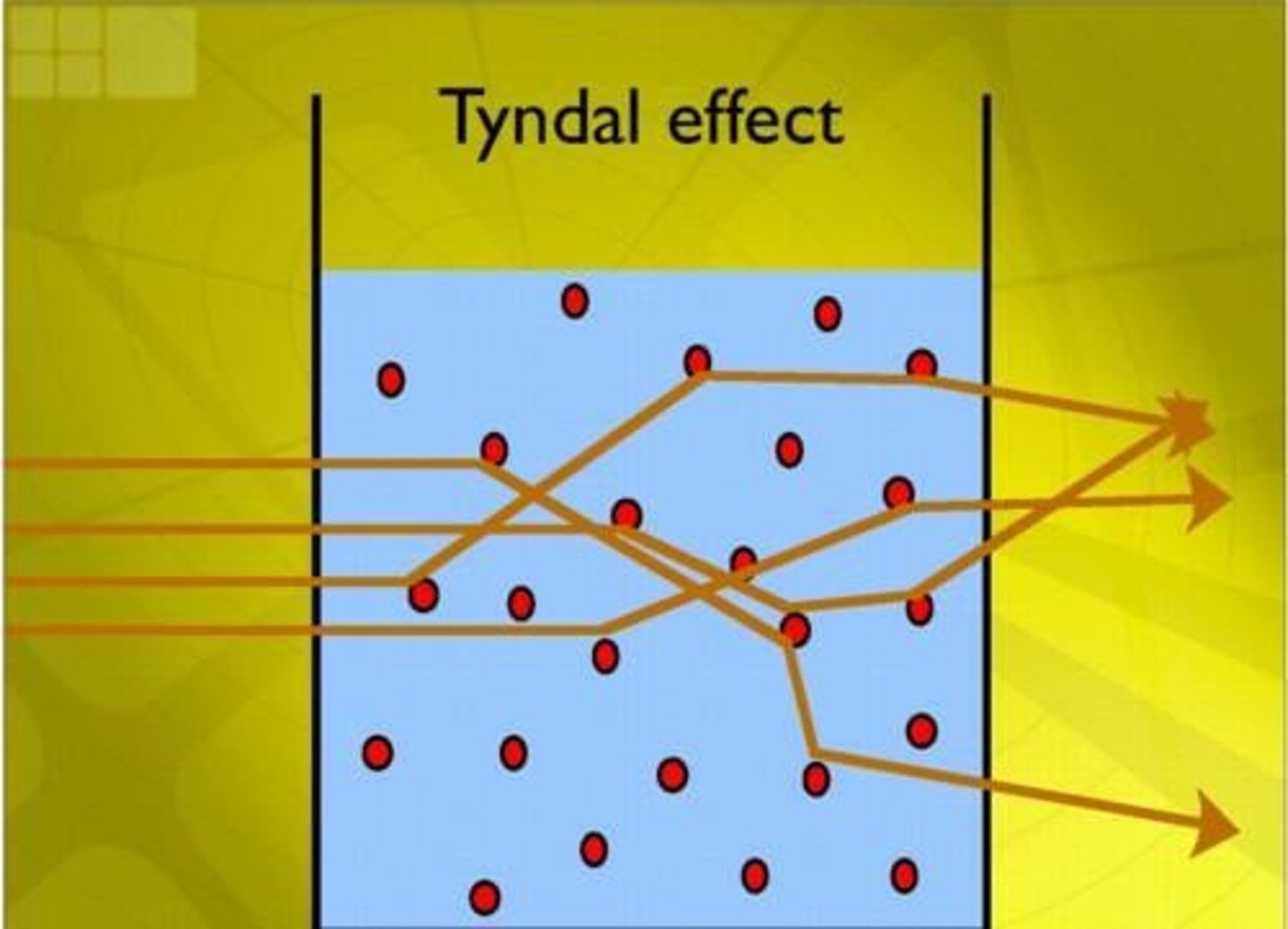
Tyndall Effect
The scattering of light by colloidal particles, allowing the beam of light to be seen.
Strong Electrolyte
A solute that completely dissociates into ions in solution, resulting in high conductivity.
Weak Electrolyte
A solute that partially dissociates into ions in solution, resulting in slight conductivity.
Non-electrolyte
A solute that does not dissociate into ions in solution, resulting in no conductivity.
Molarity (M)
The number of moles of solute per liter of solution.
Molality (m)
The number of moles of solute per kilogram of solvent.
Percent by Mass
A concentration unit calculated as (mass of solute / (mass of solute + mass of solvent)) x 100%.
Mole Fraction (X)
The ratio of the moles of a component to the total moles of all components in a mixture.
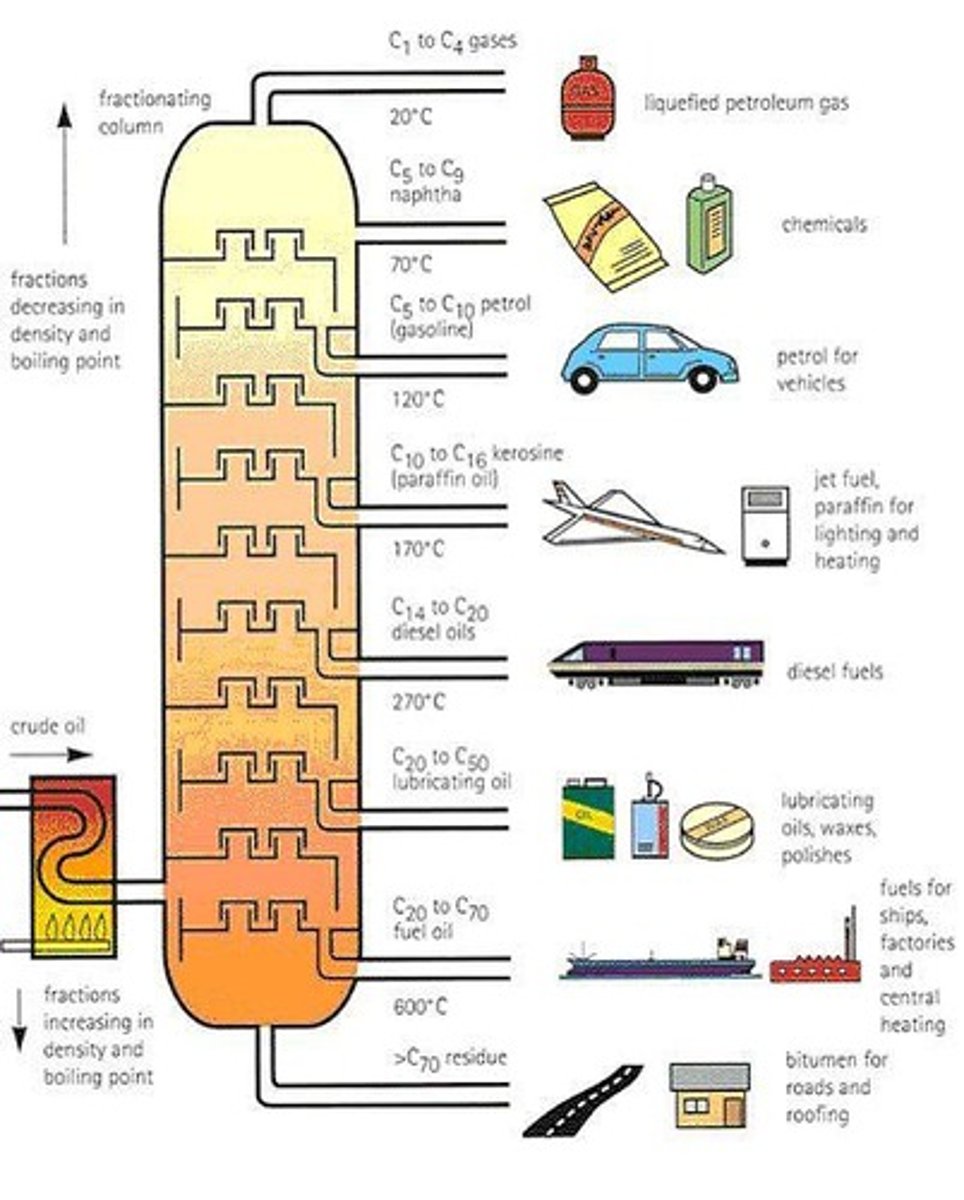
Fractional Distillation
A process used to separate homogeneous mixtures based on different boiling points.
Homogeneous Mixture
A mixture that has a uniform composition throughout.
Heterogeneous Mixture
A mixture that does not have a uniform composition and the individual components can be seen.
Air
An example of a solution that is a homogeneous mixture of gases.
Salt Water
An example of a solution that is a homogeneous mixture of salt and water.
Metal Alloys
An example of a solution that is a homogeneous mixture of metals.
Brownian Motion
The random movement of colloidal particles suspended in a fluid.
Coagulation
The process where colloidal particles clump together and may settle out.
Concentration of Solutions
The amount of solute present in a given quantity of solvent or solution.
Crude Oil
A homogeneous mixture that can be separated using fractional distillation.
Example of Molarity Calculation
What is the molarity of a solution prepared by dissolving 15.0 g of sodium hydroxide in enough water to make a total of 225 mL of solution?
Example of Molality Calculation
Calculate the molality of a solution with 8.53 g of benzene dissolved in 20.6 g of carbon tetrachloride.
Benzene
A solute with a molar mass of 78 g/mol, calculated to have 0.109 mol in 8.53 g.
Carbon Tetrahedral
A solvent with a mass of 20.6 g, converted to 0.0206 kg.
Mass/Mass
A method of expressing concentration based on the mass of solute and solvent.
Moles/Moles
A method of expressing concentration based on the ratio of moles of solute to moles of solvent.
Moles/L
A method of expressing concentration based on the number of moles of solute per liter of solution.
Moles/Mass
A method of expressing concentration based on the number of moles of solute per mass of solvent.
Preparing a Solution
The process of creating a solution by mixing solute and solvent.
Volumetric Flask
A laboratory glassware used to prepare precise volumes of solutions.
Nickel (II) Nitrate Hexahydrate
A compound to be prepared in a 50 ml of a 2M solution.
Copper (II) Chloride Dihydrate
A compound to be prepared in a 100 ml of a 1.5M solution.
Sucrose
A compound to be prepared in a 250 ml of a 0.50M solution.
Iron (II) Chloride Tetrahydrate
A compound to be prepared in a 500 ml of a 0.10M solution.
Potassium Permanganate
A compound to be prepared in a 1000 ml of a 0.25M solution.
Molality
A measure of concentration defined as moles of solute per kilogram of solvent.
Molarity
A measure of concentration defined as moles of solute per liter of solution.
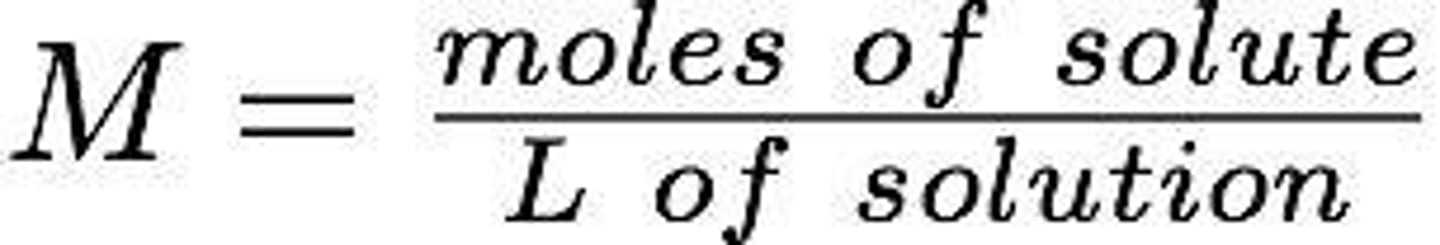
Ethanol Solution
A 5.86 M solution with a density of 0.927 g/mL, resulting in a molality of 8.92 m.
Dilution
The process of reducing the concentration of a solution by adding more solvent.

M1V1 = M2V2
The equation used to calculate the volumes needed for dilutions.
MgSO4 Dilution Example
To prepare 200 mL of 0.60M MgSO4 from a 3.0M stock solution, take 40 mL of stock and add 160 mL of distilled water.
KI Dilution Example
To prepare 250.0 mL of 0.760M KI from a 4.00M stock solution, take 47.5 mL of stock and add 202.5 mL of water.
NaCl Dilution Example
To prepare 250 mL of 0.20M NaCl using a 1.0M solution, take 50 mL of stock and add 200 mL of water.
Solvation
The process where solvent molecules surround and interact with solute particles.
Dissolution
The process of solute particles being pulled apart and entering solution.
Enthalpy Change (ΔH)
The energy change associated with the interactions broken or formed during solvation.
Ion-Dipole Interactions
Forces that allow ionic salts to dissolve in water by overcoming lattice energy.
Endothermic Process
A process that absorbs heat from its surroundings, such as the dissolution of NH4NO3 in water.
Exothermic Process
A process that releases heat to its surroundings, such as the dissolution of NaOH in water.
Enthalpy of Solution (ΔHsoln)
The change in enthalpy when a solute dissolves in a solvent, which can be either positive or negative.
ΔHsoln (MgSO4)
The enthalpy of solution for magnesium sulfate, which is -91.2 kJ/mol, indicating an exothermic process.
ΔHsoln (NH4NO3)
The enthalpy of solution for ammonium nitrate, which is 26.4 kJ/mol, indicating an endothermic process.
Entropy
A measure of disorder or randomness in a system, which can lower the energy of the system.
Dissolution
A physical change where a solute dissolves in a solvent, allowing the original solute to be recovered by evaporating the solvent.
Saturated Solution
A solution where the solvent holds as much solute as is possible at that temperature, in dynamic equilibrium with solid solute particles.
Unsaturated Solution
A solution where less than the maximum amount of solute for that temperature is dissolved in the solvent.
Supersaturated Solution
A solution that holds more solute than is normally possible at that temperature, which is unstable and can crystallize.
Dynamic Equilibrium
A state in a saturated solution where the rate of dissolving equals the rate of recrystallization.
Testing for Saturation
Throwing a crystal of solute into the solution to observe if it dissolves, remains unchanged, or causes crystallization.
Solubility Curve
A graph that shows the number of grams of a substance that can be dissolved in water at various temperatures.
Positive Slope in Solubility Graphs
Indicates that as temperature increases, more solute dissolves, typical for most solids.
Negative Slope in Solubility Graphs
Indicates that as temperature increases, less gas dissolves, typical for gases.
Saturated Solutions on Solubility Curves
Points on the traces of solubility curves represent saturated solutions at specific temperatures.
Supersaturated Solutions on Solubility Curves
Points above the traces of solubility curves represent supersaturated solutions.
Unsaturated Solutions on Solubility Curves
Points below the traces of solubility curves represent unsaturated solutions.
Sodium Acetate Solution
A solution used in heat packs that can create a supersaturated solution.
Crystallization
The process of forming solid crystals from a solution, which can be stimulated in supersaturated solutions.
Physical Change
A change that does not alter the chemical composition of a substance, such as dissolution.
Heat Absorption
The process of a system taking in heat from its surroundings, characteristic of endothermic reactions.
Heat Release
The process of a system giving off heat to its surroundings, characteristic of exothermic reactions.
Concentration
The amount of solute present in a given volume of solution, affecting saturation levels.
Solubility of gases
Increases as temperature decreases.
Cold soda
Has more bubbles due to higher gas solubility.
Hot soda
Is flat due to lower gas solubility.
Solubility of solid solutes
Increases with increasing temperature.
Carbonated soft drinks
Are more 'bubbly' if stored in the refrigerator.
Warm lakes
Have less O2 dissolved than cool lakes.
Dissolving sugar faster
Can be achieved by stirring, adding sugar to warm tea, or grinding sugar to a powder.
Unsaturated solution
Can dissolve more solute.
Saturated solution
Contains the maximum amount of solute that can dissolve.
Supersaturated solution
Contains more solute than can typically dissolve at that temperature.
Like dissolves like
Polar substances tend to dissolve in polar solvents, while nonpolar substances dissolve in nonpolar solvents.
Ethanol
Has the formula CH3CH2OH and is soluble in water due to hydrogen bonding.
Glucose
Is very soluble in water due to hydrogen bonding.
Cyclohexane
Is not soluble in water as it only has dispersion forces.
Vitamin A
Is soluble in nonpolar compounds like fats.
Vitamin C
Is soluble in water.
Solubility of gases in water
Increases with increasing mass.
Pressure effect on solubility
The solubility of a gas in a liquid is directly proportional to its pressure.
Henry's Law
Sg = kPg, where Sg is the solubility of the gas, k is the Henry's law constant, and Pg is the partial pressure of the gas.
Crude oil
Is a homogeneous mixture separated using fractional distillation.
Aqueous solution
Is a solution in which water is the solvent.
Electrolyte
A substance whose aqueous solution forms ions and conducts electricity.
Nonelectrolyte
A substance that does not form ions in an aqueous solution and is a poor conductor.
Dissociation in water
Occurs when ionic compounds separate into their component ions.
Ionization
Occurs in molecular solutes where ions first have to form and then separate in aqueous solution.
Molecular compounds
Nonmetal + nonmetal.