BioChem 285 Final
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
How does the ETC work?
- Complex III- electron flow: complex I, CoenzymeQ, Complex III, Cytochrome C, Complex IV, oxygen (final electron acceptor)
- Complex I: NADH deposits 2 electrons into complex where they are passed along a chain of redox centers (clusters of atoms with different affinities for electrons; bottom redox center has higher electron affinity and difference between redox centers is ideal for electron jumps to occur), small amount of energy released each time electron passes to the next redox center - this energy is harnessed by the complex and used to pump protons, last redox center in this complex donates 2 electrons to Coenzyme Q
- Coemzyme Q: molecules from Complex I and II donate electrons to complex III
- Complex II: similar to Complex I - electrons enter Complex II via FADH2, Complex II also transfers electrons between redox centers before donating them to Coemzyme Q, but Complex II does not use the energy liberated to pump protons
- Complex III: one electron is recyclable and passes through Complex III again later, but the other passes through 2 redox centers before reaching Cytochrome C
- Cytochrome C: carries electron to Complex IV
- Complex IV: ETC ends here where a series fo reactions involving 4 electrons converts a molecule of oxygen to 2 molecules of water; proton gradient is strengthened because 4 protons from the matrix are incorporated into H2O molecules and another 4 are pumped into the intermembrane space
What happens to the ETC is O2 is not present?
- electron transfer comes to a halt
- ATP synthesis stops
- we breathe oxygen to serve as the final electron acceptor at the end to the ETC
Favorable Enzymatic Reaction:
- exergonic (energy releasing)
- free energy of reactants is greater than free energy of products
- negative delta G
- requires activation energy to occur
Unfavorable Enzymatic Reaction:
- endergonic (taking in energy)
- free energy of products is greater than free energy of reactants
- positive delta G
- requires activation energy to occur
- requires coupling
Which of the following statements is true?
A) An enzyme drives an unfavorable reaction forward by lowering ∆G
B) An enzyme drives favorable reactions forward by lowering ∆G
C) An enzyme cannot change the thermodynamics of a reaction.
D) Enzymatic reactions that require coupling always have a net negative ∆G after coupling
E) The presence of an enzyme inhibitor will change the ∆G of the reaction.
- an enzyme cannot change the thermodynamics of a reaction
- enzymatic reactions that require coupling always have a net negative delta G after coupling
What is one way that an effector could work either as an inhibitor or activator?
- binding of an allosteric activator would trigger a conformational change in protein where the active site is accessible to substrate
- binding of an allosteric inhibitor would bind and change the conformation fo the protein such that the active site is now inaccessible to the substrate
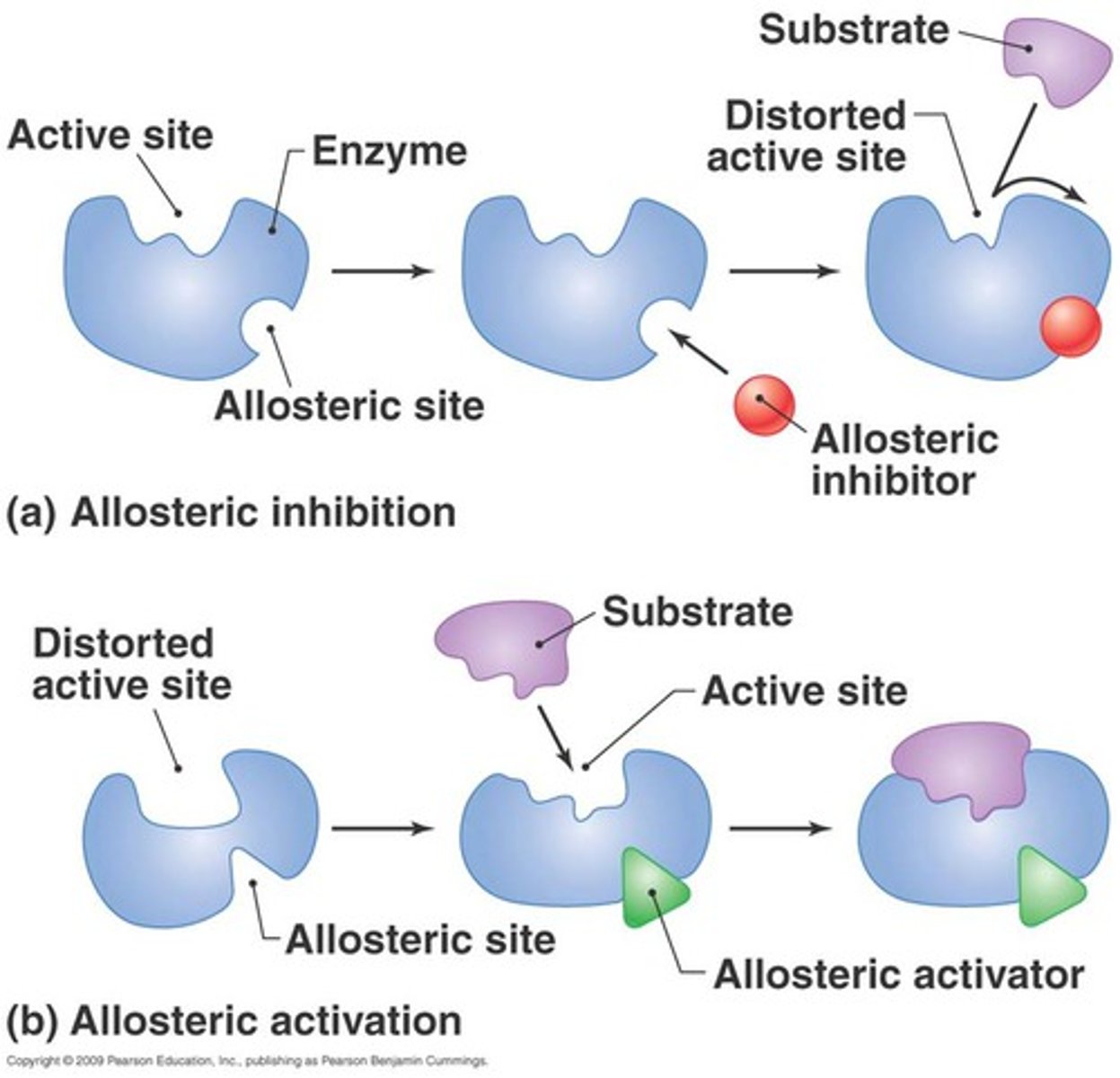
Which of the following if the most accurate way to describe how feedback inhibition works in metabolism?
A) end-product of the pathway acts as an allosteric inhibitor of an enzyme at the beginning of the pathway.
B) The end-product of the pathway acts as a competitive inhibitor that binds the active site of the enzyme responsible for its own production.
C) The metabolic pathway is shut-down by the lack of an intermediate enzyme.
D) Inhibitors bind to the active site of an enzyme at the beginning of a pathway.
E) Production of the end-product is inhibited by a metabolic intermediate from a different pathway.
- end product of the pathway acts as an allosteric inhibitor of an enzyme at the beginning of the pathway
Which of the following would be the best example of feedback inhibition?
- GMP (product) inhibits PRPP synthetase (one of the 1st enzymes in the pathway)
- prevents a wasted energy as it stops the pathway within the first few steps of its progression
Cells store energy for immediate use in which of the following?
A) NADH
B) glucose
C) ATP
D) pyruvate
E) glycogen
- NADH
- ATP
- glucose is broken down into pyruvate which is then broken down into energy
- glycogen is used for energy storage
Which reactions would be considered anabolic?
A) The reaction between acetyl-CoA and oxaloacetate to produce citrate in the TCA cycle
B) Conversion of pyruvate to Acetyl-CoA for the TCA cycle
C) Conversion of pyruvate to phosphoenolpyruvate in gluconeogenesis (opposite of glycolysis)
D) Conversion of Pyruvate to ethanol
E) The entire TCA cycle
- reactions between acetyl-CoA and oxaloacetate to produce citrate in the TCA cycle (taking 2 reactants and forming 1 product)
- conversion of pyruvate to phosphoenolypyruvate in gluconeogenesis (opposite of glycolysis which breaks glucose down)
Why is there more energy in NADH and FADH, compared to ATP?
- when NADH and FADH2 donate their electrons to the ETC, enough protons are pumped into the intermembrane space to provide the energy for the creation of 2-3 ATP (for NADH) or 1-2 ATP (FADH2) by ATP synthase
- these electron carriers provide the energy to pump protons into the cell to create the proton gradient which drives ATP synthase
What does it mean when a molecule is "reduced?"
- a molecule is reduced when it gains negatively charged electrons from its electron donor
- reduced molecules typically have lost bonds with oxygen and gained bonds with hydrogen
A molecule with a positive standard reduction potential will also be...
- a good electron acceptor
- an oxidizing agent (is reduced)
- reduced in a redox reaction (accepts electrons)
NADH is primarily produced and used in catabolic pathways, whereas NADPH is primarily used in anabolic pathways - Why is this?
- having different electron carriers for different pathways helps to confer specificity on a chemical reaction and provides a means for regulation
Triose phosphate isomerase converts DHAP to G3P in glycolysis - What would happen if triode phosphate isomerase were mutated to be non-functional?
- if DHAP were not converted to G3P, then only half of the original glucose molecule would be converted to pyruvate (less efficient)
What is the enzyme responsible for the commitment step of glycolysis? Under what conditions is this enzyme most active - Why?
- phosphofructokinase (PFK)
- PFK is most active when ATP concentrations are low and glycolysis needs to run to create more ATP (energy)
Which of the following is true of glycolysis?
A) 2 ATP are put in and a net gain of 4 ATP are produced
B) 2 NAD+ are put in and 4 NADH are generated
C) 1 NADH is put in and 1 NAD+ is generated
D) No NAD+ is generated
E) 2 moles of pyruvate are generated per mole of glucose
- no NAD+ is generated (problem of glycolysis)
- 2 moles of pyruvate are generated per mole of glucose
Which of the following is correct about NADH?
A) Compared to NADPH and FADH2, it has the lowest reduction potential
B) It contains phosphate bonds
C) It is a coenzyme
D) It is generated through fermentation pathways
E) It is generated through the TCA cycle
- compared to NADPH and FADH, it has the lowest reduction potential (worst electron acceptor)
- it contains phosphate bonds
- it is a coenzyme
- it is generated through the TCA cycle (tot hen be oxidized to NAD+)
The TCA cycle occurs primarily in which of the following places?
A) The cytosol of a eukaryotic cell
B) The cytosol of a prokaryotic cell
C) the mitochondrial matrix of a eukaryotic cell
D) the intermembrane space of a eukaryotic cell
- the cytosol of a prokaryotic cell
- the mitochondrial matrix of a eukaryotic cell
T/F: Acetyl-CoA is both a substrate and product of the TCA cycle
- False: it is only a substrate; it is not regenerated in the TCA cycle
T/F: Oxaloacetate is both a substrate and a product of the TCA Cycle
- True: oxaloacetate feeds in and is regenerated in the last step of the TCA cycle
Which of the following goes into the TCA cycle?
A) 5 reduced energy electron carriers
B) 3 CO2
C) 3 NAD+
D) 1 ADP equivalent
E) 1 FAD
- 3 NAD+
- 1 ADP equivalent (GDP)
- 1 FAD
What are the products of the TCA cycle?
- 1 GTP
- 3 NADH
- 1 FADH2
- 2 CO2
For mitochondria that are active in respiration, indicate whether to movement of each of the following molecules into (I) or out of (O) the matrix is thermodynamically favorable
- (I): O2, H, ADP
- (O): CO2
Which of the following is one of the 3 main steps in aerobic respiration?
A) High energy electrons power ATP-producing turbines
B) PMF extracts high energy electrons
C) PMF is charged by ATP-producing turbines
D) High energy electrons are extracted from substrates
E) All of the above
- high energy electrons are extracted from substrates
Aerobic respiration is said to occur in 3 steps (1 - glycolysis, 2 - TCA, 3 - ETC/ATP synthase) - categorize each of the following with the correct "step" of aerobic respiration:
A) high energy electrons are extracted from substrates
B) Pyruvate is generated
C) High energy electrons are used to create PMF
D) PMF powers ATP producing turbines
E) ATP is used to generate molecules with increased bond energy
- (A) higher energy electrons are extracted from substrates (2)
- (B) Pyruvate is generated (1)
- (C) High energy electrons are used to create PMF (3)
- (D) PMF powers ATP producing turbines (3)
- (E) ATP is used to generate molecules with increased bond energy (1)
First/Primary Messenger:
- the first signaling component in a pathway, typically coming from outside of the cell
- purpose: to relay information from the extracellular environment to the cell
- Ex: insulin, nitric oxide, testosterone
Second Messenger:
- a small, non-protein molecule used to amplify signals intracellularly
- Ex: cAMP, DAG, IP3, Ca2+
Signal Transduction:
- the mechanisms by which signals are sensed, relayed and integrated into cellular pathways so that an appropriate response to environmental conditions can be made
- Ex: glucagon signaling pathway
How does a kinase regulate target proteins?
- Kinase: protein that phosphorylates target proteins (on tyrosine, serine, and threonine residues)
- phosphorylation changes tha conformation of the target protein and can activate or inhibit its function
- phosphatase: opposite of a kinase - will dephosphorylate target proteins (activating or inhibiting as well)
The inactive, heterotrimeric G protein is initially bound to ... and not associated with the receptor
- GDP
A ... binds to the receptor, the heterotrimeric G protein associates with the receptor and ...
- signal molecule/primary messenger/hormone
- GDP is released
A ... then binds to the ... subunit and activates it, and the ... subunits stay associated with each other and are activated as well.
- GTP
- alpha
- beta and gamma
What scenario would most likely occur if the GPCR shown was inhibited?
- if epinephrine could not bind, adenylyl cyclase would not be activated and the calcium channel would stay closed
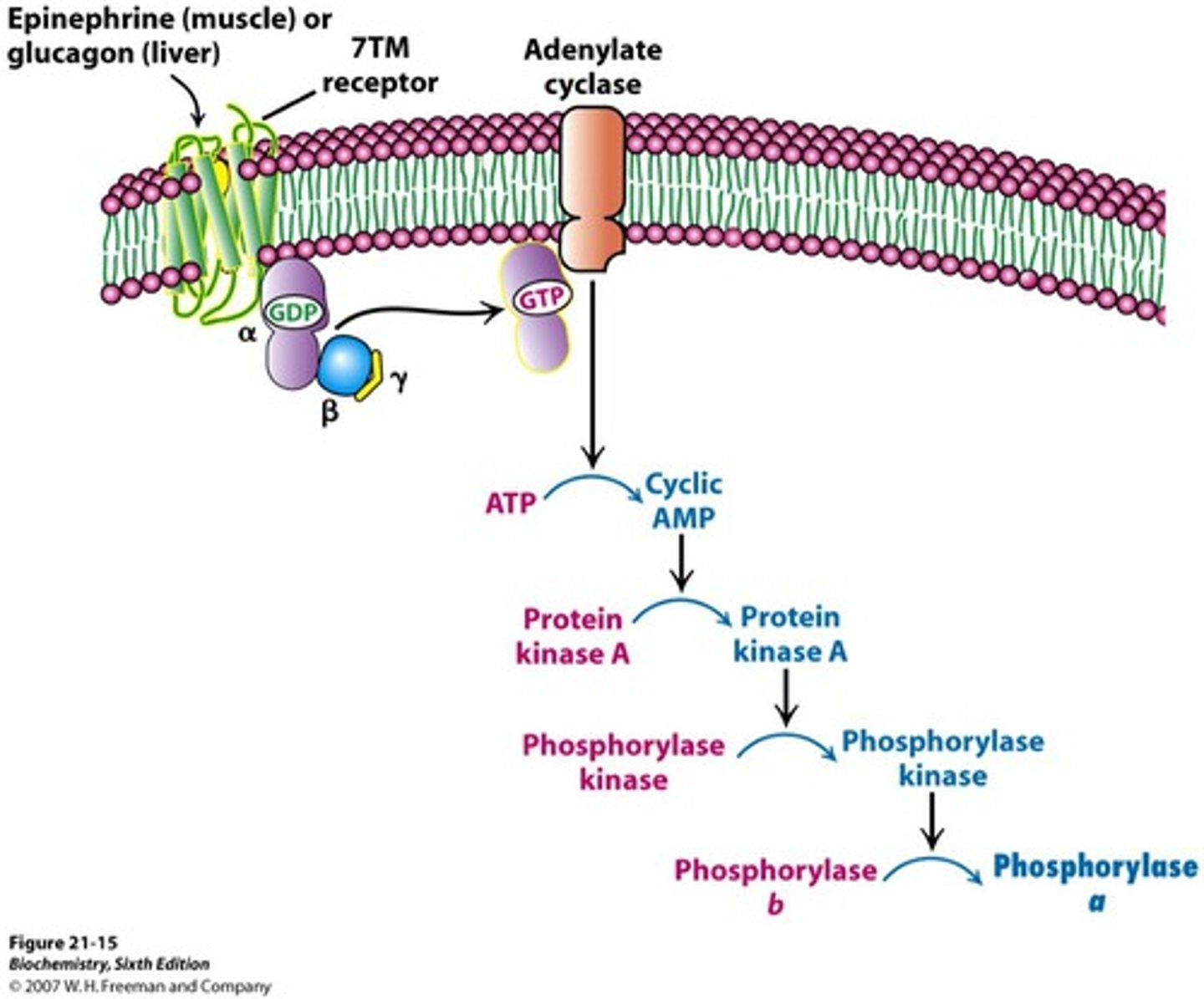
Glucagon is recognized by a liver cell with a glucagon receptor. Which of the following is a downstream event that would happen as a result?
The pathways leading to the mobilization of glucose are activated
Glycogenesis and glycolysis are both activated
Upregulation of conversion of PEP to Pyruvate
Downregulation of gene expression regulated by CREB
- the pathways leading to the mobilization of glucose are activated (high glucagon concentration in the bloodstream indicates that the cell needs energy)
T/F: 2 cAMP molecules bind to each regulator subunit of PKA
- true
- cAMP is a second messenger which activates the PKA to phosphorylate other proteins
T/F: PKA phosphorylates CREB in response to glucose influx via SLC2A1
- false
- KREB is phosphorylated in response to glucagon being recognized by the glucagon receptor which stimulates ACDY to produce cAMP
T/F: Binding of GTP to G alpha triggers dissociation from the GPCR and stays associated with the G beta/gamma subunits
- false
- G alpha also dissociates from G beta/gamma
When adrenaline (epinephrine) binds to adrenergic receptors (a GPCR) on the surface of a muscle cell, it activates a G-protein, initiating a signaling pathways that result in the breakdown of muscle glycogen (a storage polymer composed of glucose molecules) to glucose (its monomer component); this gives muscle cells a burst of energy (muscle cells do not respond to glucagon as that hormone is more specific to liver cells. How would you expect glycogen breakdown to be affected if muscle cells were injected with a non-hydrolyzable analog of GTP which cannot be converted to GDP?
- Explain what would happen if non-hydrolyzable GTP were added to cells in the absence of adrenaline?
- Each time a G protein picks up a non-hydrolyzable analog of GTP it would be locked into tis active form in which it would stay since it would not be able to escape by the usual route of GTP hydrolysis
- In the absence of adrenaline, most of the G protein would initially be in the GDP-boudn (inactive) form and GDP is released only slowly in the absence of stimulation by an activated receptor
- Thus, you might expect a slow activation of G protein after injection of a non-hydrolyzable GTP analog and a correspondingly slow increase in glycogen breakdown as the GDP is slowly released without the help of an active receptor
Explain what would happen if non-hydrolyzable GTP were added to cells after a brief exposure to adrenaline.
- In the presence of adrenaline, GDP would be rapidly released from the G protein (because the adrenergic receptor - GPCR - binds signal) and non-hydrolyzable GTP would be bound in place of the GDP as the receptor is activated
- A brief exposure to adrenaline would normally stimulate glycogen breakdown for a short time until adrenaline was removed in turn, turning the signaling pathway off
- However, in the presence of a non-hydrolyzable analog of GTP, the pathway would remain on even after adrenaline was removed.
- Thus, the non-hydrolyzable analog would cause a prolonged response to a pulse of adrenaline as the receptor is turned on and never turned off through ATP hydrolysis
The Gas/MAPK pathway regulates cellular proliferation. Alterations in this pathway often lead to cancer development. Of the options below, select the mutation that is most likely to lead to cancer.
A) A gain of function mutation in Ras GAP
B) A loss of function mutation in of Ras GAP
C) A mutation where SOS is unable to bind to GRB2
D) A mutation in GRB2 that prevents binding to an activated RTK
E) A loss of function mutation in MEK (MAP Kinase Kinase)
- a loss of function mutation in Ras GAP
Which of the following would serve to down regulate the Gas/MAPK pathway?
A) An overactive Ras GEF
B) An overactive Ras GAP
C) A kinase that positively regulates Raf
D) A kinase that negatively regulates MEK (remember inhibitory phosphorylation?)
E) A phosphatase that dephosphorylates active MAPK
- an overactive Was GAP (GTP-GDP)
- a kinase that negatively regulates MEK (negative regualtion-inhibition)
- a phosphatase that dephosphorylates active MAPK (active-inactive)
In a receptor tyrosine kinase, the activation loop of the intracellular kinase domain changes conformation upon:
- phosphorylation of a tyrosine residue within the loop
Imagine you want to identify a drug that indices apoptosis in cancer cells. Based on the pathway below which of the following would be a promising drug type to pursue?
- a small molecule that inhibits BCK2 (this molecule inhibits a regulatory molecule signaling for the apoptosis pathway so inhibiting this molecule would prevent apoptosis inhibition)
What are 2 classes of molecular switches most often used in signaling pathways?
- GTP binding proteins: Ras (GTPase) activates targets in a pathway when it is bound to GTP - can be switched on by a GEF which triggers GDP release and subsequent GTP binding and can be turned off by a GAP which stimulates GTPase activity in Ras
- Heterotrimeric G-proteins (associated with GPCRs) operate in the same way as Was because they are activated by GTP binding and inactivated by GDP binding
- Protein Phosphorylation: Raf (a kinase) activates targets when it is phosphorylated and also activates other kinases bu phosphorylating them - Raf can be switched off by phosphorylation or dephospho rylation events (like all other kinases); others include MEK, MAPK
Given what you know about insulin and glucagon signaling, which of the following statements are true?
A) when blood glucose is high, insulin will activate gluconeogenesis (in liver)
B) when blood glucose levels are low, glucagon will activate glycogen degradation (in liver)
C) when insulin is produced, glucagon will be inhibited
D) Insulin will signal for activation of phosphofructokinase (PFK)
E) Only the insulin pathway will use kinases for signal transduction.
- when blood glucose levels are low, glucagon will activate glycogen degradation (in liver) - insulin indicates that we need more energy in the cell from the high levels of glucose in the bloodstream; indicates that the cell should take up glucose
- when insulin is produced, glucagon will be inhibited; insulin opposes glucagon which signals when blood glucose levels are low
- Insulin will signal for activation of phosphofructokinase (PFK) which functions in glycolysis pathway as we have a lot of glucose in the blood stream and should use it to make energy
In the image of the following lamellipodium what statements are false?
A) Nucleation of new filaments near the leading edge pushes the plasma membrane forward.
B) ARP proteins are sufficient to effectively nucleate the branched actin filaments in the lamellipodium
C) Capping proteins are added to the plus end, and thus prevent filament elongation.
D) There is more ADP-bound actin at the leading edge than in the actin filaments away from the leading edge.
- ARP proteins are sufficient to effectively nucleate the branched actin filaments in the lamellipodium - need Formin for nucleation
- There is more ADP-bound actin at the leading edge than in the actin filaments away from the leading edge - the leading edge contains more ATP bound actin
Which of the following statements about actin is false?
A) ATP hydrolysis decreases actin filament stability.
B) Actin is largely localized around the cell periphery, and helps govern the shape of the plasma membrane.
C) Actin filaments are nucleated by formin in lamellipodia, and thus have a linear structure.
D) The cell signaling coordinates actin filaments polymerization during cell movement.
- Actin filaments are nucleated by formin in lamellipodia, and thus have a linear structure - actin filaments in lamellipodia have a branched structure
Compared to the normal situation, in which actin monomers carry ATP, what do you predict would happen if actin monomers that bind a nonhydrolyzable form of ATP were incorporated into actin filaments?
- actin filaments would grow longer because ATP hydrolysis is what causes filaments to fall off and without this, there would be a constant growth
What of the following statements regarding dynamic instability is false?
A. Each microtubule filament grows and shrinks independently of its neighbors.
B. The GTP cap helps protect a growing microtubule from depolymerization.
C. GTP hydrolysis by the tubulin dimer promotes microtubule shrinking.
D. The newly freed tubulin dimers from a shrinking microtubule can be immediately
captured by growing microtubules and added to their plus end.
- The newly freed tubulin dimers from a shrinking microtubule can be immediately
captured by growing microtubules and added to their plus end - although alpha subunit is always bound to GTP, the beta subunit is not always and it must be to result in a growing MT
Which of these situations will enhance MT shrinkage?
A. Addition of a drug that inhibits GTP exchange on free tubulin dimers
B. addition of a drug that inhibits hydrolysis of the GTP carried by tubulin dimers
C. addition of a drug that increases the affinity of tubulin molecules carrying GDP for other tubulin molecules
D. addition of a drug that blocks the ability of a tubulin dimer to bind to γ-tubulin
- Addition of a drug that inhibits GTP exchange on free tubular dimers - free tubulin dimers are interested in MT growth and shrinkage
For both actin and MT polymerization, nucleotide hydrolysis is important for...
- decreasing the binding strength between subunits on filaments - filaments can be addend, but once they are added they can also fall off so their intereations cannot be too strong
T/F: The hydrolysis of ATP is what motivates the "power Stoke during myosin movement.
- False - the release of the Pi is what motivates the Power Stroke or movement as this causes a weaker association between the MT and dyne which allows the motor protein to move forward toward the minus end
T/F: Upon binding a MT, the leading head of Kinesin releases ADP for ATP. ATP binding in the leading head drives the movement of the second head of kinesin and allows kinesin movement to progress.
- True - the ATP hydrolysis and release of Pi allows the trailing head to be bound to the MT more weekly to allow the head to move forward; when ATP comes in and binds to leading head, which allows a stronger association, this causes the trailing head to be able to fly forward
T/F: If the GTP concentration in a cell is extremely low, then a MT is more likely to assemble ends as opposed to disassemble
- False - MT require ATP to assemble as beta tubulin must be associated with ATP to assemble
The plus and minus ends of a MT and/or actin filament are recognized by these motor proteins - How?
- like the filament itself, the individual monomers/dimers that make up the filament have directionality
- Motor proteins recognize this directionality by binding to specific sites on the filament.
- Ex: Dynein - binds between the grooves of the alpha and beta subunits of tubulin
Dynein walks alone ... in the ... direction
- MT
- minus
Myosin crawls along ... in the ... direction
- actin
- plus
Kinesin walks along ... in the ... direction
- MT
- plus (except for Kinesin-14)
What would you expect to see in Kinesin function along a MT with a nonhydrolyzable form of ATP?
- Kinesin woudl bind, but not walk along MT.
- The first head would bind this ATP form which would induce a conformational change that swings the second head forward, but the first head would not be able to dissociate from the MT as the ATP form is not able to be hydrolyzed which is what allows the Kinesin to dissociate
- The shape of the ATP form is similar enough to ATP that the kinesin heads would be able to bind to it, but since hydrolysis of ATP by the head is necessary to release it from the MT, the heads would not be able to unbind from the MT which would prevent Kinesin from being able to continue walking along the MT