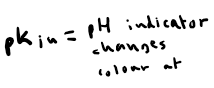Topic 12: Titration curves + pH
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
How do you measure pH?
Universal indicator (paper/solution)
pH meter (machine)
What are the pros and cons of the universal indicator?
Pros: more convenient than pH meter
Cons: Doesn’t work really well sometimes, colour might not match scale, very subjective
What are the pros and cons of the pH meter?
Pros: More accurate as it gives pH to more sig figs than universal indicator
Cons: Requires frequent calibration for reliable readings
How do you calibrate the pH meter?

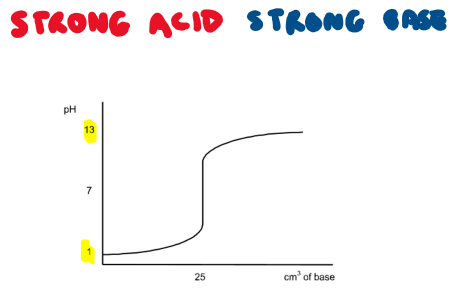
Draw the titration curve for a strong acid + strong base reaction
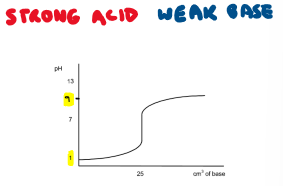
Draw the titration curve for a strong acid + weak base reaction
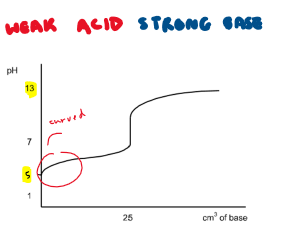
Draw the titration curve for a weak acid + strong base reaction
Draw the titration curve for a weak acid + weak base reaction
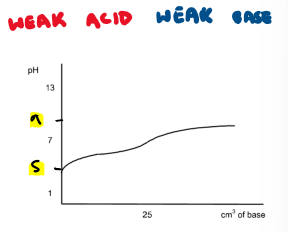
What is the equivalence point?
point where thing in burette neutralises thing in flask

How do you calculate the equivalence point
What is the half-neutralisation point?
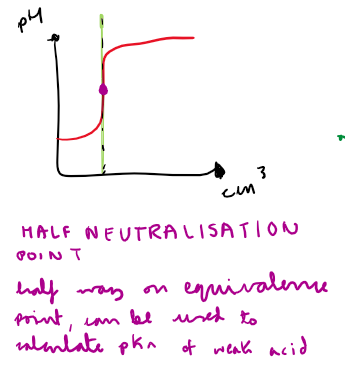
How do you use the half-neutralisation point to calculate the pH of the weak acid?
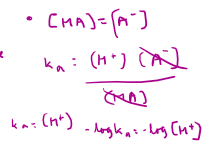
How do you choose an indicator for a titration curve?

When looking at the data table for a suitable indicator, what does pKin tell you?