AP Biology - Big Ideas from Unit #3: Cellular Energetics
1/68
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
Cell's Metabolism
Chemical reactions that take place within cells.
Ex. Anabolism, Catabolism.
Metabolic Pathway
A series of chemical reactions that either builds a complex molecule (anabolic reaction) or breaks down a complex molecule into simpler compounds (catabolic reaction).
Anabolic Reaction (Anabolism)
A reaction that builds new molecules and/or stores energy (Ex. Dehydration Synthesis)
REQUIRES ENERGY
Catabolic Reaction (Catabolism)
A reaction that breaks down molecules into small ones. (Ex. Hydrolysis)
RELEASES ENERGY (DOES NOT REQUIRE ENERGY)
Kinetic Energy
Energy due to motion
Potential Energy
Energy referred to the potential to do work.
Free Energy
Measure of Energy available to do work
Chemical Energy
Potential energy existing within chemical bonds; released when bonds are broken.
Gibbs Free Energy
Usable Energy; Energy available to do work - AKA Free Energy
Exergonic Reaction
A chemical reaction that releases energy.
Ex. Catabolic Reactions
THINK: EXergonic = Energy EXiting.
Endergonic Reaction
A chemical reaction, in which free energy is absorbed from the surroundings (REQUIRE INPUT OF ENERGY).
Ex. Anabolic Reactions
THINK: ENderonic = Energy ENters.
Activation Energy
Small amount of energy input needed for all chemical reactions to occur.
Enzyme
A type of protein that speeds up a chemical reaction (acts as a biological catalyst) in a living thing.
Function of Enzymes
Speed up chemical reactions by lowering activation energy.
Explain how enzymes speed up chemical reactions
Enzymes function as catalysts to speed up chemical reactions by lowering the initial amount of energy needed to begin the reaction.
Enzymes are usually made of _________.
Enzymes are usually made of Proteins.
T/F: Are enzymes reusable?
TRUE: Enzymes are reusable.
NAMING: Enzymes usually end with the suffix _____.
-ase.
Enzymes usually end with the suffix -ase.
Active site of Enzyme
The region of an enzyme where substrate molecules bind and undergo a chemical reaction.
Substrate
The reactant on which an enzyme works; binds to active site.
Explain why enzymes usually facilitate only one type of reaction.
"STRUCTURE DETERMINES FUNCTION!"
Due to each enzyme's unique structure, it can only facilitate one specific type of reaction, as its unique structure determines its unique function.
T/F: The active site of the enzyme is specific to the substrate it binds with.
TRUE: The active site of the enzyme is specific to the substrate it binds with as the enzymes unique structure determines its unique function, meaning it can only facilitate specific reactions.
In the context of enzymes, explain the type of relationship substrate concentration has with the reaction rate of the enzymes.
THINK: Substrate Concentration and Reaction Rates have a _________ relationship.
In the context of enzymes, substrate concentration and the speed of the reaction rate have a direct relationship, meaning:
⬆️ Substrate Concentration = ⬆️ Speed of Reaction Rate
--AND--
⬇️ Substrate Concentration = ⬇️ Speed of Reaction Rate
The greater the concentration of substrates, the faster the reaction rate; the same is true if the concentration of substrates is decreased.
Competitive Inhibition
A process by which a chemical substance has a shape that fits the active site of an enzyme and competes with the substrate for the active site, effectively inhibiting the enzyme.
BIG IDEA: Active Site blocked by Competitive Inhibitor.
Noncompetitive Inhibition (aka Allosteric Inhibition)
Describes when the inhibitor binds with the enzyme at a site other than the active site (known as the allosteric site) and inhibits the enzyme by altering the shape of the active site.
BIG IDEA: The active site is modified due to a noncompetitive inhibitor acting on the allosteric site, making it difficult for the substrate to fit into the active site.
Does competitive and noncompetitive inhibition affect the reaction rate of the enzyme? Explain.
Yes, competitive and noncompetitive inhibition affect the reaction rate of the enzyme as they both affect the substrate concentration by decreasing it, thus slowing down the reaction rate of the enzyme.
Denaturation of Enzyme
Occurs when the enzyme is outside the "optimal range" and the enzyme's shape changes making it now less effective or ineffective)
Changes in the tertiary structure of the enzyme.
Environmental Factors that can lead to Enzyme Denaturation
1) Change in Temperature (Increasing Heat outside of Optimal Range)
2) Change in pH
3) Exposure to metal ions
Explain how denaturation affects Enzyme Function
The Catalytic (Catalysis) ability of the enzyme is lost/significantly decreased, making the enzyme less efficient. However, in some cases, the affects of denaturation are reversible.
Note: Denaturation only affects the active site; NOT THE WHOLE ENZYME.
Quick Note about Cellular Respiration & Photosynthesis
Unit #3 is a huge unit, covering enzymes, cellular respiration, photosynthesis, and molecular fitness (2020 CED only).
Cellular Respiration & Photosynthesis might seem daunting but remember this: The AP Biology Exam and Curriculum do not expect you to know all the details of these complex pathways--only the location, inputs (not the exact number, but the exact number would be great!), outputs, and the significance/why of it. Don't believe me? Check the CED on CB's website.
Keep that in mind while studying. Know the Big Picture/Idea. These cards reflect the big picture/idea of the concepts and are meant to serve as a review. If you need to learn these complex processes, you are better off reading your textbook or watching YT Lectures.
I hope these help!
~Sahil
Aerobic Organisms
Organisms that require oxygen for survival.
THINK: Aerobic → Oxygen Present
Anaerobic Organisms
Organisms that do not require oxygen for survival.
THINK: Anaerobic → Oxygen Absent
Cellular Respiration
A process that releases energy by breaking down glucose and other food molecules in the presence of oxygen.
CED (paraphrased from CollegeBoard): "Cellular respiration in eukaryotic organisms consists of a sequence of coordinated enzyme-driven processes that harness energy from biological macromolecules."
The products of Cellular Respiration include 6CO₂, 6H₂O, and ATP! The inputs of Cellular Respiration include Glucose (C₆H₁₂O₆) and 6CO₂.
The Chemical Formula for Cellular Respiration is...
C₆H₁₂O₆ + 6CO₂ → 6CO₂ + 6H₂O + ATP.
Note that you aren't required to memorize this equation; you just need to know the big picture.
IDENTIFY the 4 key stages of Cellular Respiration (Aerobic)
1) Glycolysis
2) Oxidation of Pyruvate (aka Link reaction)
3) Krebs Cycle
4) Oxidative Phosphorylation (Electron Transport Chain & Chemiosmosis)
Honorable Mention: Fermentation
THINK: "G → P → K → O" = Glycolysis → Pyruvate Oxidation → Krebs Cycle → Oxidative Phosphorylation. Fermentation steps in only if O₂ bails out.
Differentiate between Aerobic & Anerobic Respiration - What processes occur?
Aerobic → Glycolysis, Oxidation of Pyruvate, Krebs Cycle, Oxidative Phosphorylation
Anaerobic → Glycolysis & Fermentation
THINK: With oxygen, you can do all the stages of Cellular Respiration. Without oxygen, you can only do Glycolysis & Fermentation
NADH & FADH₂
High Energy Electron Carriers that deliver electrons to the ETC.
Oxidized Forms: NADH & FADH₂
Reduced Forms: NAD+ & FAD+
Oxidation
Loss of electrons.
THINK: "LEO: Loss of Electronsis Oxidation" OR "OIL: Oxidation is Loss"
Reduction
Gain of Electrons
THINK: "GER: Gain Electrons is Reduction" OR "RIG: Reduction is gain"
Mnemonics to differentiate between Oxidation & Reduction
a) "LEO GER" → Loss of Electrons is Oxidation AND Gain of Electrons is reduction.
b) "OIL RIG" → Oxidation is LOSS AND Reduction is GAIN
Pick one of these mnemonics! They're incredibly helpful, not just in AP Biology but in Chemistry as well. If you took chemistry previously, you likely have a favorite.
Glycolysis
A biochemical pathway that releases energy from glucose.
CED (paraphrased from CollegeBoard): "Glycolysis is a metabolic pathway that converts energy stored in glucose into ATP by utilizing ADP and inorganic phosphate, produces NADH from NAD+, and results in the formation of pyruvate."
Know the following:
a) Location: Cytosol
b) Inputs: 2 NAD+, 2 ATP, and, 1 Glucose Molecule (C₆H₁₂O₆).
c) Outputs: 2 (net) ATP (4 ATP produced, 2 used), 2 NADH, and 2 pyruvate (3 Carbon Molecules)
d) Oxygen Required? No. Occurs in both aerobic and anaerobic organisms.
e) Why It Matters: Glycolysis is the first step of cellular respiration and occurs in all organisms — with or without oxygen. It provides cells with a quick supply of ATP and generates pyruvate and NADH, which fuel further energy extraction in aerobic conditions. Its universality and cytoplasmic location highlight its evolutionary importance.*
*This connects to Unit #7, when we're discussing Common Ancestry.
Oxidation of Pyruvate
Pyruvate, which is generated from glycolysis, undergoes oxidation, causing the NAD+ electron carrier to be reduced to NADH. During the oxidation of the pyruvate molecule, one carbon is released as CO2, resulting in a remaining 2-carbon acetyl group. This 2-carbon acetyl group is then linked to Coenzyme A, creating Acetyl-CoA, which is sent to the Krebs cycle.
Know the following:
a) Location: Mitochondria
b) Inputs (both from Glycolysis): Pyruvate (3-carbon) and NAD+
c) Outputs: Acetyl-CoA (2-carbon), Carbon Dioxid (1-carbon), and NADH
d) Oxygen Required? Not directly but required for continuation of the process. Aerobic Respiration only.
e) Why It Matters: Pyruvate cannot enter the Mitochondrial Matrix, it must be converted into Acetyl-CoA for the Krebs Cycle.
Coenzyme A (CoA)
Coenzyme A (CoA) is a non-protein organic coenzyme that binds to and transports acetyl groups during cellular respiration, forming acetyl-CoA, the molecule that enters the Krebs Cycle.
a) In Cellular Respiration: In the oxidation of pyruvate reaction, a 3-carbon pyruvate is decarboxylated → 2-carbon acetyl group. CoA binds the acetyl group → forms acetyl-CoA.
Acetyl-CoA delivers the 2-carbon unit into the Krebs Cycle for further oxidation.
b) Functional Role: Acts like a carrier molecule: it doesn't provide energy but enables the acetyl group to enter the cycle. Essential connector between glycolysis and the Krebs cycle.
c) Why It Matters: No CoA = No acetyl-CoA = No Krebs Cycle.It's the molecule that physically ushers carbon into the mitochondrial spotlight for full oxidation and ATP generation.
THINK: "CoA is the chauffeur. Pyruvate's carbon group doesn't enter the Krebs Cycle without a ride." It escorts the carbon into the energy economy.
Krebs Cycle (aka the Citric Acid Cycle)
During the citric acid cycle, the two carbon atoms of acetyl CoA are oxidized, releasing CO2. The energy released during these reactions produces ATP, NADH, and FADH2. Most energy initially stored in the glucose molecule is in the high-energy electrons carried by NADH and FADH2.
CED (paraphrased from CollegeBoard): "In the Krebs cycle, carbon dioxide is produced from organic compounds, ATP is generated from ADP and inorganic phosphate, and electrons are delivered to the coenzymes NADH and FADH2."
Know the following:
a) Location: Mitochondrial Matrix
b) Inputs: Acetyl-CoA (2-Carbon), 3 NAD+, 1 FAD+, 1 ADP + inorganic phosphate
c) Outputs (per 1 turn of the cycle): 2 Carbon Dioxides (1-Carbon each), 3 NADH, 1 FADH2, and 1 ATP (produced via substrate-level phosphorylation)
d) Oxygen Required? Yes. Aerobic Respiration.
e) Why It Matters: The Krebs Cycle is a central hub for electron carrier generation. These high-energy electrons are essential for the electron transport chain in oxidative phosphorylation.
Oxidative Phosphorylation
Oxidative Phosphorylation is the final step of aerobic cellular respiration, where most ATP is produced. It involves the Electron Transport Chain (ETC) and Chemiosmosis, using high-energy electrons to pump protons (forming an electrochemical proton gradient) and drive ATP synthesis via ATP synthase (protons from the proton gradient run down it).
CED (paraphrased from CollegeBoard): "Electrons that are released during glycolysis and the Krebs cycle are carried by NADH and FADH2 to the electron transport chain located in the inner mitochondrial membrane. As electrons move through the ETC in a series of reactions, a proton (hydrogen ion) electrochemical gradient is created across the inner mitochondrial membrane."
Know the following:
a) Location: Inner mitochondrial membrane (Mitochondrial Cristae)
b) Inputs: 10 NADH, 2 FADH₂, 6O₂ (final electron acceptor), ADP + inorganic phosphate
c) Outputs: 26-28 ATP, 6 H₂O, Regenerated NAD⁺ and FAD (fed back into earlier stages)
d) Oxygen Required? Yes, absolutely, as oxygen serves as the final/terminal electron acceptor. Without it, the ETC halts and ATP synthesis collapses.
e) Why It Matters: Oxidative phosphorylation produces the majority of ATP in aerobic organisms, powering nearly all cellular functions. It also shows how gradients (H⁺ concentration) can be harnessed to drive energy-requiring processes — a key concept in cellular energetics. The need for oxygen links this step to respiration, circulation, and even evolutionary fitness.
Decoupling Oxidative Phosphorylation
Decoupling occurs when a protein or molecule allows protons (H⁺) to leak across the inner mitochondrial membrane without passing through ATP synthase. This disrupts the electrochemical gradient, meaning the ETC still pumps protons, but ATP synthesis is reduced or halted.
Instead of making ATP, the energy from the proton gradient is released as heat. This can increase an organism's metabolic rate and generate warmth, especially useful in hibernating mammals or infants (e.g., brown fat tissue with uncoupling proteins).
CED (paraphrased from CollegeBoard): "In aerobic cellular respiration, decoupling oxidative phosphorylation from electron transport produces heat. Endothermic organisms can utilize this heat to maintain their body temperature."
Why It Matters: Decoupling shows that electron transport and ATP production are not inseparable. It provides a mechanism for thermoregulation and demonstrates flexibility in metabolic pathways — essential in extreme environments.
THINK: "Protons take the shortcut." Imagine the mitochondria trying to charge a battery (ATP), but someone pokes a hole in the circuit — energy leaks out as heat instead. Great if you're cold, bad if you need ATP.
Electron Transport Chain
Protons are pumped into the mitochondrial Inner Membrane Space and generate an electrochemical proton gradient. The final electron acceptor is Oxygen.
THINK: Protons pumped by channel proteins, proton gradient formed, terminal electron acceptor: oxygen
Chemiosmosis
ATP synthase utilizes that electrochemical proton gradient, established by the electron transport chain, and has protons run down that concentration gradient, synthesizing ATP.
Fermentaton
Anaerobic pathway that regenerates NAD⁺ when oxygen is absent. Purpose: Regenerate NAD+ when oxygen's not available.
CED (paraphrased from CollegeBoard): "Fermentation enables glycolysis to continue without oxygen and generates organic substances, such as alcohol and lactic acid, as byproducts.
Lactic Acid Fermentation
Lactic acid fermentation is an anaerobic process that allows glycolysis to continue in the absence of oxygen by regenerating NAD⁺ from NADH. It occurs in muscle cells, certain bacteria, and some fungi.
Know the following:
a) Location: Cytosol (same as glycolysis)
b) Organisms: Animal cells (esp. muscle), some bacteria (e.g., Lactobacillus)
c) Inputs: Pyruvate, NADH
d) Outputs: Lactic acid (lactate), NAD⁺ (regenerated)
e) Oxygen Required? No. Anaerobic Respiration.
f) Why It Matters: Lactic acid fermentation temporarily sustains ATP production (via glycolysis) when oxygen is unavailable. It ensures that NAD⁺ is recycled, allowing continued energy production. This is especially critical during intense muscle activity or in anaerobic environments.
Alcohol Fermentation
Alcohol fermentation is an anaerobic process carried out by yeast and some bacteria, allowing glycolysis to continue by regenerating NAD⁺ and producing ethanol and CO₂ as byproducts.
Know the following:
a) Location: Cytosol
b) Organisms: Yeast, some prokaryotes
c) Inputs: Pyruvate, NADH
d) Outputs: Ethanol, CO₂ (gas released — causes bread to rise), NAD⁺
e) Oxygen Required? No. Anaerobic Respiration.
f) Why It Matters: Alcohol fermentation enables ATP production in anaerobic environments, critical for unicellular organisms and has major biotechnological applications — brewing, baking, biofuels. Like lactic acid fermentation, it regenerates NAD⁺, ensuring glycolysis can continue.
Photosynthesis
Process by which photoautrotrophs utilize light energy to convert water and carbon dioxide into oxygen and high-energy carbohydrates such as sugars and starches.*
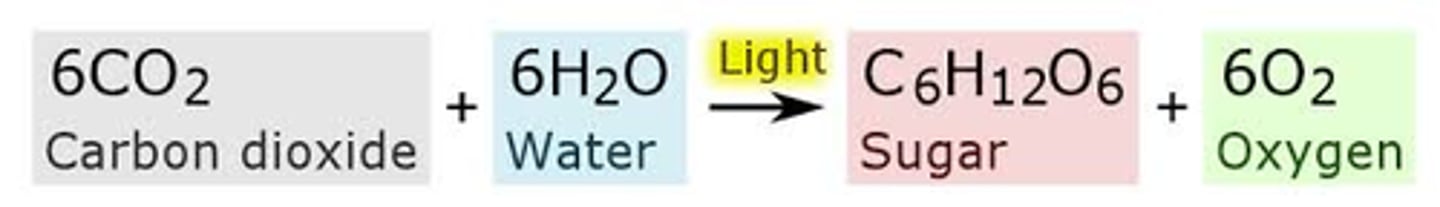
The chemical equation for photosynthesis is
6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂. Over the arrow should be light energy.
CED (paraphrased from CollegeBoard): "Photosynthesis involves a sequence of processes that use carbon dioxide, water, and solar energy to create carbohydrates and oxygen. Organisms that perform photosynthesis harness energy from sunlight to generate sugars that can be utilized in biological functions or reserved for later use."
*This connects to Unit #8, when we're discussing autotrophs and their impact on trophic levels and ecosystems.
Who performs photosynthesis?
Plants, Algae, and Photosynthetic Bacteria (like cyanobacteria!).
Connections between Evolution & Photosynthesis
Photosynthesis first evolved in prokaryotic organisms such as cyanobacteria, releasing oxygen, and may have triggered the first mass extinction event. These prokaryotic photosynthetic pathways served as the foundation for the photosynthesis pathways found in modern-day multicellular organisms.*
CED (paraphrased from CollegeBoard): "Photosynthesis initially developed in prokaryotic organisms. Scientific data supports the assertion that prokaryotic (cyanobacterial) photosynthesis contributed to the creation of an oxygen-rich atmosphere. The photosynthetic pathways of prokaryotes laid the groundwork for photosynthesis in eukaryotes."
*This connects to Unit #7, when we're discussing evolution.
Identify who performs Cellular Respiration & Photosynthesis.
All organisms perform cellular respiration but not photosynthesis--only plants, algae, and photosynthetic bacteria perform photosynthesis.
IDENTIFY the 2 main parts of photosynthesis
1) Light-Dependent Reactions
2) Light-Independent Reactions (Calvin Cycle)
EXPLAIN how the Light-Dependent Reactions and Calvin Cycle are Interdependent
The light-dependent reactions use light energy to produce ATP and NADPH, which are then used as energy and electron sources in the Calvin Cycle to convert carbon dioxide into glucose. In turn, the Calvin Cycle regenerates ADP, inorganic phosphate, and NADP⁺, which are necessary inputs for the light-dependent reactions to continue. This reciprocal exchange of molecules ensures both processes are sustained, demonstrating their biochemical interdependence.
Location of Photosynthesis in Plants
Occurs within the Chloroplasts.
The Light Dependent Reactions occur in the thylakoid membrane. The Calvin Cycle (Light-Independent Reactions) occurs within the stroma.
Location of Photosynthesis in Photosynthetic Bacteria
In prokaryotic organisms that undergo photosynthesis, the reactions dependent on light happen on invaginations of the plasma membrane, while the reactions that do not require light take place in the cytosol.
Chlorophyll
A pigment that absorbs light and harnesses the energy from sunlight. The main light-absorbing pigments involved in photosynthesis are chlorophylls. Chlorophylls are present in photosystems I and II (PSI and PSII).
Photosystem
A photosystem consists of proteins, chlorophyll, and various light-absorbing pigments known as accessory pigments. PSI and PSII have distinct types of chlorophyll that capture the highest light energy at slightly varying wavelengths (700 nm for PSI and 680 nm for PSII). Photosystems are found in the thylakoid membrane of the chloroplast and are linked by an electron transport chain (ETC).
Photolysis
A reaction taking place in the thylakoid membranes of a chloroplast during light-dependant reactions where two molecules of water are split to form oxygen, hydrogen ions, and electrons
Calvin Cycle Steps
1) Carbon Fixation
2) Reduction
3) Regeneration of RuBP
Carbon Fixation
Fixation refers to the process of converting a biologically inactive form into one that can be utilized. In the process of carbon fixation, the Rubisco enzyme attaches a single molecule of carbon dioxide to the five-carbon compound ribulose-bisphosphate (abbreviated as RuBP). This reaction leads to the formation of a six-carbon intermediate that is unstable and subsequently splits into two three-carbon molecules.
Reduction in the Calvin Cycle
The ATP and NADPH generated during the light-dependent reactions are utilized to convert the three-carbon molecules. The energy required for this process is supplied by ATP, while NADPH contributes the hydrogen atoms, serving as the reducing agent. At the conclusion of this process, a three-carbon compound known as glyceraldehyde-3-phosphate (G3P) is formed. G3P can be utilized for sugar synthesis, although a portion of it is allocated for the final stage of the Calvin cycle: regeneration.
RuBP Regeneration
To allow the Calvin cycle to proceed, the regeneration of the five-carbon molecule RuBP is essential. Every set of five G3P molecules (which are three-carbon compounds) contributes a total of 15 carbon atoms. Utilizing ATP generated from the light-dependent reactions, these five G3P molecules are rearranged to produce three molecules of RuBP (a five-carbon compound), which also holds 15 carbon atoms. This transformation necessitates energy, derived from the light-dependent reactions.
Light Reactions - Big Picture
Absorbs light energy and uses electron transport chain to produce NADPH & ATP!
Know the following:
a) Location: Thylakoid Membrane
b) Inputs: Water (electrons) & Lhotons (Light energy)
c) Outputs (products): ATP & NADPH
d) Why It Matters: Produces ATP & NADPH needed for Calvin Cycle
Calvin Cycle - Big Picture
Uses ATP & NADPH to yield food!
Know the following:
a) Location: Stroma
b) Inputs: 3 CO2, 9 ATP, 6 NADPH
c) Outputs: G3P (glucose)
d) Why It Matters: Produces food for cell
Linear Electron Flow (Photosynthesis)
The primary pathway, involves both photosystems and produces ATP and NADPH using light energy.
THINK: PSI & PSII, Synthesizes ATP & NADPH
Cyclic Electron Flow (Photosynthesis)
A mechanism that allows ATP synthesis without making NADPH.
THINK: PSI Only, Synthesizes ATP Only