periodic trends + bonds and polarity
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
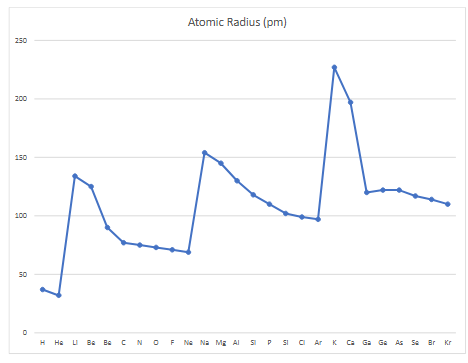
atomic radius
Distance from the nucleus to the valence shell
dependent and independent variable in AR graph
Atomic # = independent variable
Atomic radius = dependent variable
nuclear charge (Z)
positive charge present in the nucleus of an atom
keeps electrons in place
shielding
the repulsion of outer electrons by inner electrons
stronger than sideways repulsion
effective nuclear charge
Net attraction of electrons to the nucleus
Zeff = Z (nuclear charge) - shielding
sideways repulsion
repulsion between electrons on the same shell
causes spaces between electrons
weaker than shielding
mr.moniz term
trend of Zeff
across a period (left to right): protons increase while inner shielding electrons always stay the same, so effective nuclear charge also increases
down a group: a new shell is added, all inner orbits become shielding electrons, increased shielding and low inner p+:e- causes more repulsion than attraction, so effective nuclear charge decreases
trend of atomic radius
across a period (left to right): protons increasing, inner shielding electrons stay the same, so Zeff increases, causes increased attraction, electrons pulled in more closely, atomic radius decreases
down a group: AR does not increase because of an added orbit. A new valence orbit is added, inner orbits become shielding electrons, more repulsion, the p+:inner e- ratio decreases, valence is less drawn to the nucleus, atomic radius increases
ionization energy
minimum amount of energy required to remove an electron from a neutral element in the gaseous state producing a +1 cation
since minimum, it removes electron from valence shell
Xg + IE = Xg+ + e- (free electron)
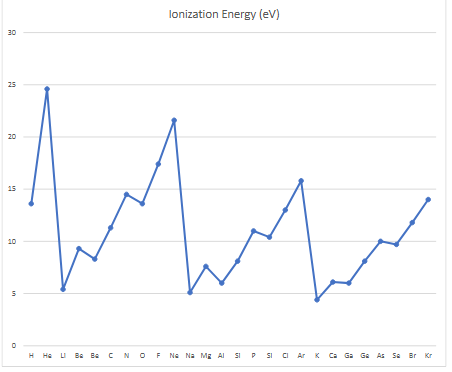
trend of ionization energy
across periods (left to right): Zeff increases, shielding stays the same while p+ increases, causing more attraction, more energy needed to remove e-, p+:inner e= ratio increasing, ionization energy increases
down a group: Zeff decreases, new shielding orbit, more repulsion than attraction, less energy required dislodge e-, p+:inner e- ratio decreases, ionization energy decreases
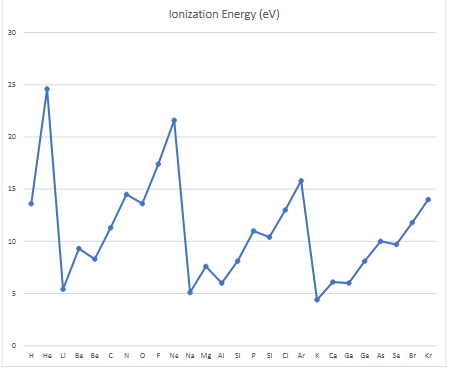
what does the spike in the ionization energy graph show?
small spikes show jumps to inner orbit
big spikes downwards show next row
more energy needed for inner electrons due to increased effective nuclear charge
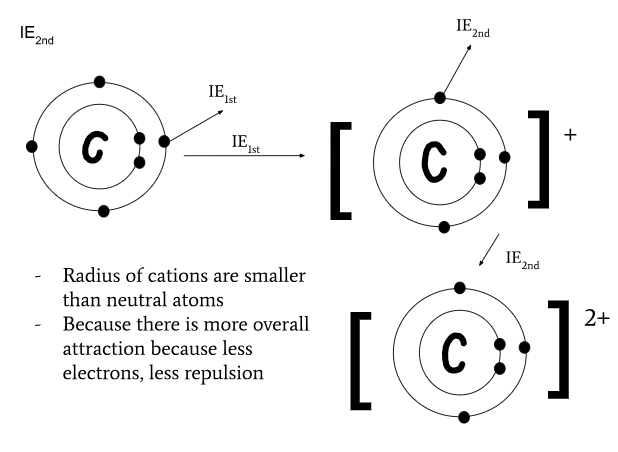
multiple ionization energies
minimum energy required to remove subsequent electron to form a cation in an element in its gaseous state
Xg+ + IE2nd = Xg2+ + e- (+2 cation and free electron)
change is discontinuous
each subsequent electron needs more energy to be removed
why is more IE needed overtime? in one element, not as a trend
Sideways repulsion is decreased because the first electron was removed from valence shell
overall repulsion decreases so the attraction is more effective
attraction itself does not increase but bc repulsion is less, overall attraction increases
electron affinity
energy released by a neutral atom in the gaseous state when it attracts an electron to form a -1 anion (negative energy)
X(g) + e- → X-(g) + E.A
why is energy releases when an electron is added to a neutral atom
the electron is attracted to the positively charged nucleus
falls into atom
the potential energy is being converted into kinetic energy
as it loses energy, that energy must be released
energy released as light once electron reaches atom, this energy is EA
what does it mean if an electron needs to be pushed into an atom (EA)?
that means there is repulsion (from the other electrons there)
repulsion to add electron: negative electron affinity written as a positive number
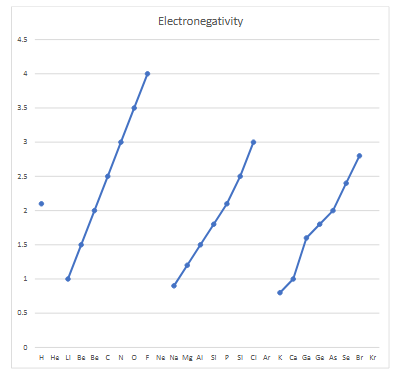
electronegativity
a calculated average of measurements indicating the tendency of an element to attract an electron charge towards it in a compound
What factors allow metallic elements to form ions where they have lost their valence electrons?
Metallic elements effective nuclear charge is low
IE is also low
The proton to inner electron ratio is lower
so metallic elements are more likely to form cations because they have lower ionization energy
stable
how difficult is something to change, is it difficult or easy to change
electron affinity
energy released by a neutral atom in the gaseous state when it attracts an electron to form a -1 anion, exothermic
electronegativity and electron affinity trend
across a period (left to right): both increase, EN: more protons, unchanged shielding, Zeff increases, more attraction, greater tendency to attract electron. EA: more energy given off when anion forms
down a group: both decrease, EN: new shielding, more repulsion than attraction, tendency to gain electron is less. EA: greater repulsion, less energy released when anion is formed
noble gasses do not attract electrons because the valence shell is full, and much more energy is needed to overcome the repulsion for an electron to go into the next shell
noble gasses have high IE and are less likely to lose an electron
all trends general
left to right: Zeff increases, AR decreases, IE increases, EN and EA increases
down a group: Zeff decreases, AR increases, IE decreases, EN and EA decreases
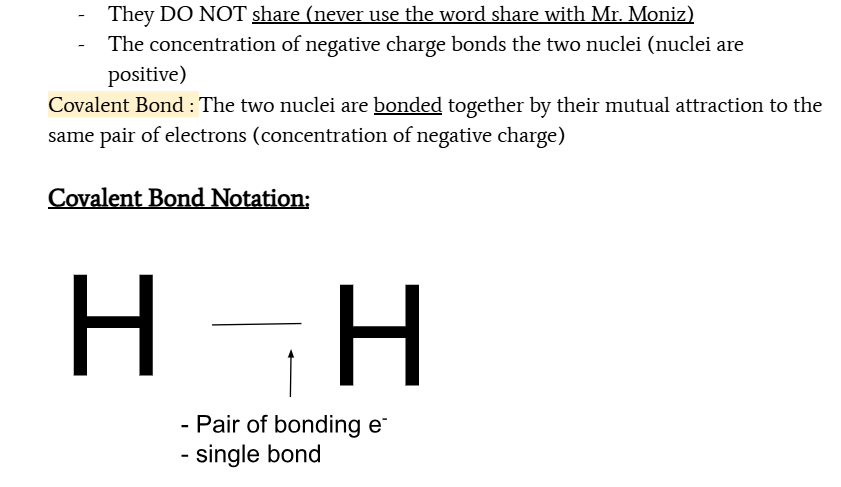
covalent bond
a bond where two nuclei of nonmetals are bonded together by their mutual attraction to the same pair of electrons
how does a covalent bond occur?
two nonmetal atoms collide so that the nuclei are closer than two times the radius
the electron pair is drawn to the internuclear distance where they can create a concentration of charge
side ways repulsion increased
mutual attraction to same pair of electron bonds atoms together
creates a covalent bond
differences between ionic and covalent bonds
ionic:
non localized and non directional
all ions pack together
forms ionic crystal lattice
covalent
attraction is localized to the internuclear distance
attraction is directional along internuclear distance
ex. in a H2 bond, another extra hydrogen will not bond as no attraction is available
forms molecule
How and why do ionic compounds form?
a non metal and metal atom must physically collide with enough energy that an electron from the metal is dislodged
the metal has low IE, easier to remove electron
the non metal has high EN and EA, the dislodged electron is attracted to the non metal
it is always true that…
丨IE丨 (energy required) > 丨EA丨 (energy released) therefore the electron transfer from metal to nonmetal always requires energy and the ions are less stable than the neutral atoms
how does an ionic crystal lattice form?
the positive and negative ions attract
every cation surrounded by a number of anions and so on to maximize attraction
a great amount of energy is required to create the crystal lattice, but much more is needed to break it apart, so it is stable
ionic compound is more stable than neutral elements due to high attraction necessary to break apart
endothermic vs exothermic
Endothermic: Process that absorbs/requires energy
Exothermic: releases energy
Why does covalent bonding result in molecules and not ionic crystal lattices?
ionic bond: metal can attract nonmetal as long as they are relatively close, it is non directional and non localized, which is why they pack together to create a crystal lattice
covalent bond: the attraction is localized and directional to the internuclear distance and not just anywhere, so ions cannot pack together
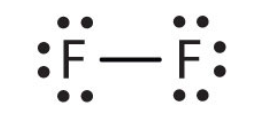
what type of bond is this?
single bond
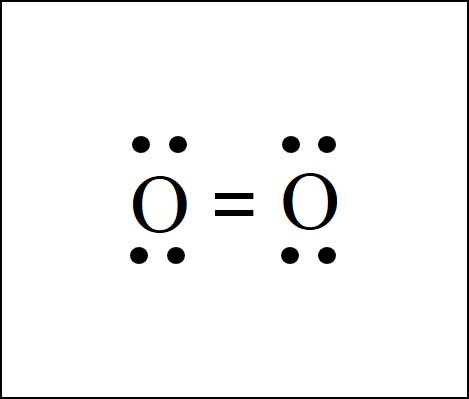
what type of bond is this?
double bond
polarity
uneven distribution of netgative charge in a bond
Partial charges: δ + or δ-
used to indicate the polarity of a covalent bond
detla positive on less EN element
detla negative on more EN element
how do you predict what kind of bond will form between elements in a compound?
use differences in electronegativity
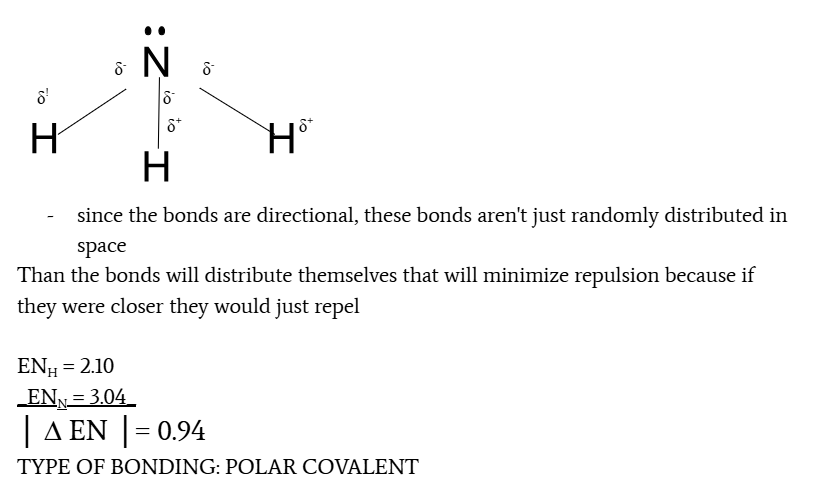
electronegativity differences and type of bond
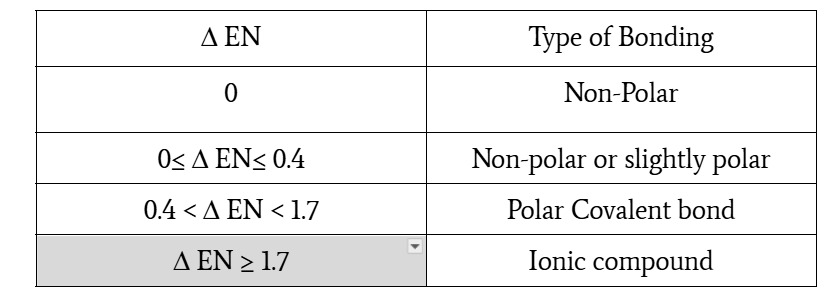
0≤ Δ EN≤ 0.4, non-polar or slightly polar
0.4 < Δ EN < 1.7, polar covalent bond
Δ EN ≥ 1.7, ionic compound
radius of cations and anions compared to their neutral atom
radius of cations are smaller than their neutral atom since there are less electrons, more overall attraction
radius of anions are greater than their neutral atom since there are more electrons, more overall repulsion