Electron Configuration
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Quantum number n
Determines the energy of the orbital; energy increases as this number increases
Quantum number I
Specifies the possible orbital shapes for a given n. For a given n, l = 0, 1, 2,… n-1.
l = 0 | s orbital (1 orbital)
l = 1 | p orbital (3 orbitals)
l = 2 | d orbital (5 orbitals)
l = 3 | f orbital (7 orbitals)
Quantum number ml
Specifies which of the orbitals corresponds to a particular electron.
ml = -l,….. -1, 0, 1,…., l
Quantum number ms
Electron spin | can take one of two values: +1/2 or -1/2
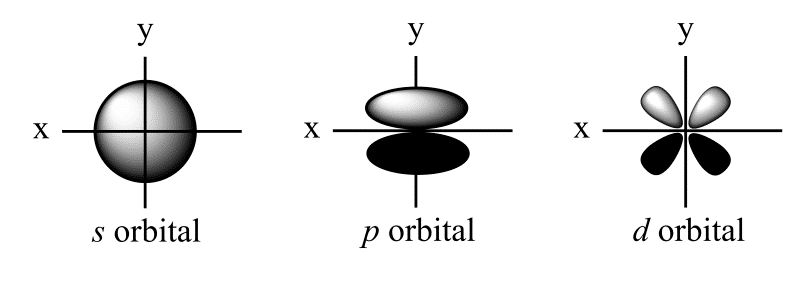
Atomic orbital shapes
s orbitals are spherical
p orbitals are dumbbell shaped; x, y, z
d orbitals are clover-shaped
The Pauli Exclusion Principle
No two electrons can have the same four quantum numbers. An orbital can hold at most two electrons, and then only if the electrons have opposite spin
Aufbau principle
The ground state configuration is determined by filling orbitals with the lowest energy
Hund’s Rule
The lowest energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals with the same spin before pairing them
Exception to the Building-up principles
Elements whose electron configuration ends in d4 become d5 (half-filled), and those with d9 become d10 by getting one electron from the last level
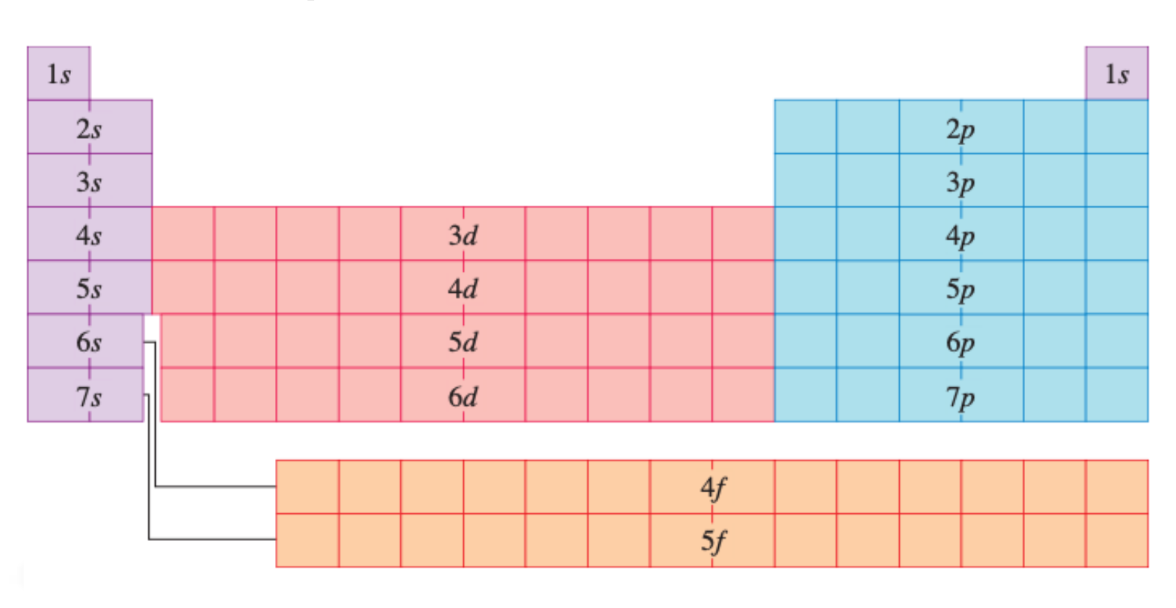
Electron configuration shortcut in periodic table
Far left; 1-7s
Middle; 3-6d
Right; 2-7p
Bottom; 4-5f
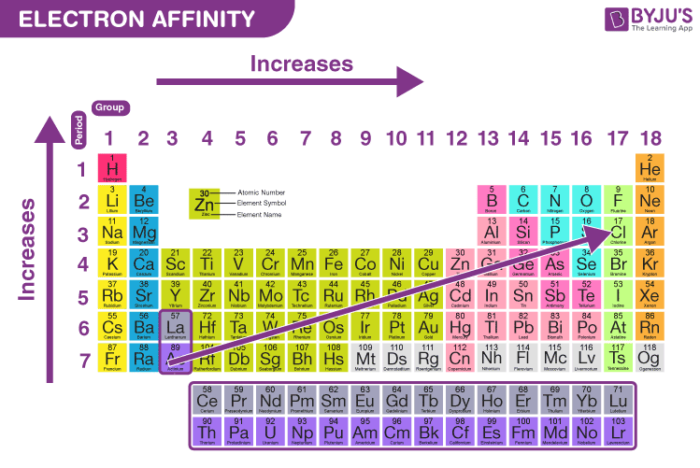
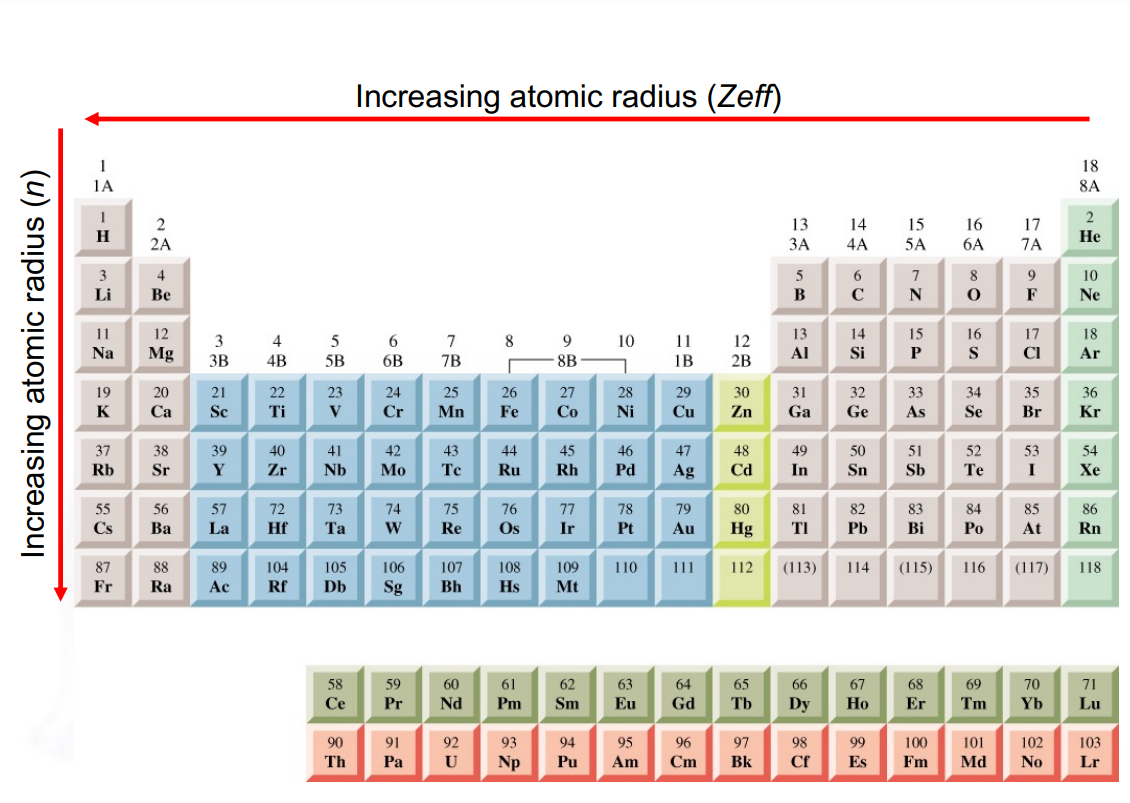
Order of increasing atomic radius
Order of increasing ionization energy
Order of increasing electron affinity