Y12 FINAL CHEM CARDS
1/460
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
461 Terms
Covalent bond
Electrostatic attraction between a shared pair of electrons and the positively charged nuclei
Octet Rule
Tendency of atoms to gain a valence shell with a total of 8 electrons
Lewis Formula
Shows all valence electrons, whether these are pairs of electrons involved in covalent bonds or non-bonded pairs of electrons (non-bonding pairs)
Bond Strength
Measure of the energy required to break a bond
- More bonds = stronger the bond
Multiple bonds = Involve greater number of shared electrons = electrostatic attraction between them and positive nuclei is stronger = stronger bond
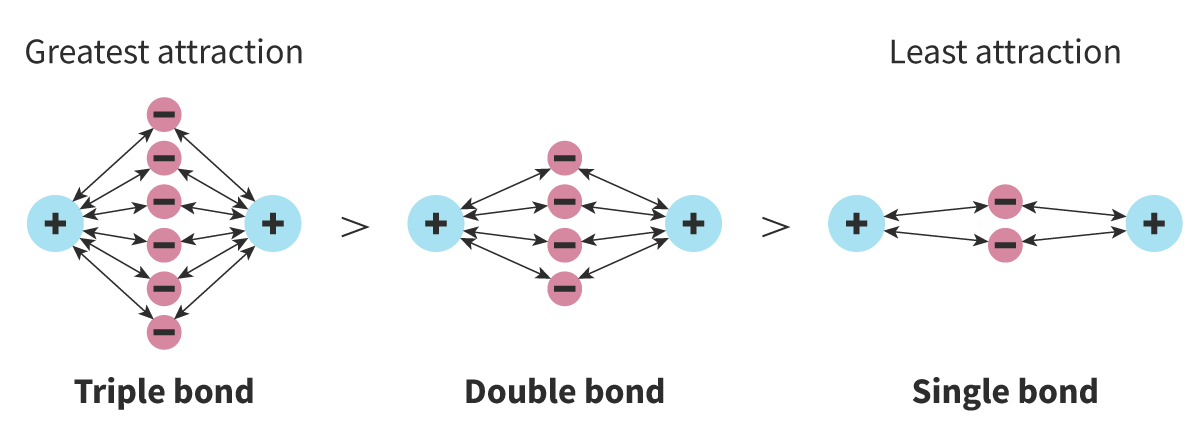
Bond Length
Measure of the distance between 2 bonded nuclei
Bond strength
C-N < C=N < C≡N
Bond length
C-N > C=N > C≡N
Coordination bonds
A covalent bond where both of the electrons being shared in the bond have been donated by one atom
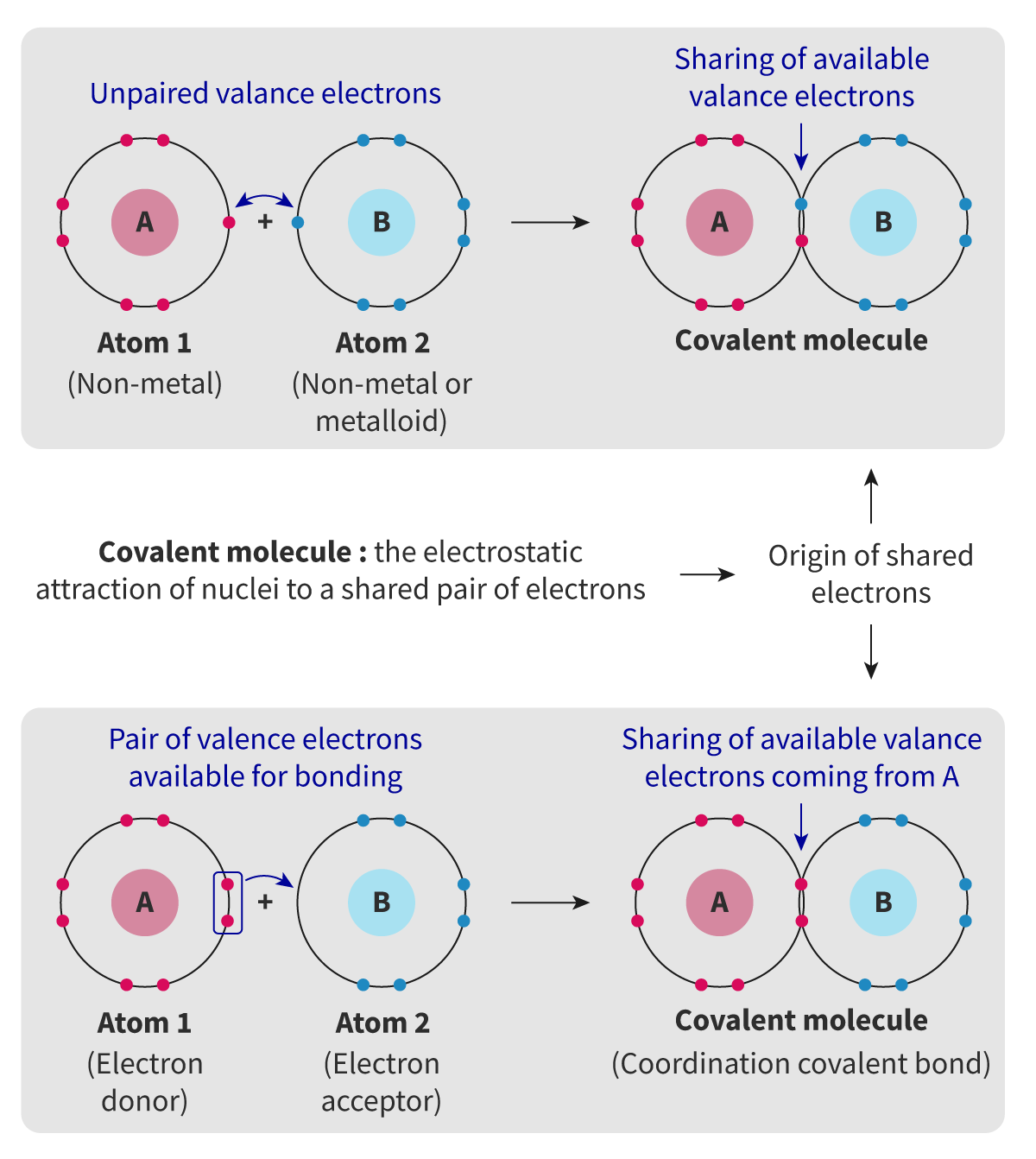
To form a coordination bond:
One of the species in the bond must have non-bonding electrons available for bonding --> This atom is called "donor"
Coordination bonds allow atoms in a molecule to achieve stable configuration with 8 electrons in outermost shell = Accordance with octet rule
To form a coordination bond:
One of the species in the bond must have ___-_____ electrons available for bonding --> This atom is called "____"
Coordination bonds allow atoms in a molecule to achieve ____ configuration with 8 electrons in ______ shell = Accordance with octet rule
Donor to Receiver
Arrow in a coordination bond points from:
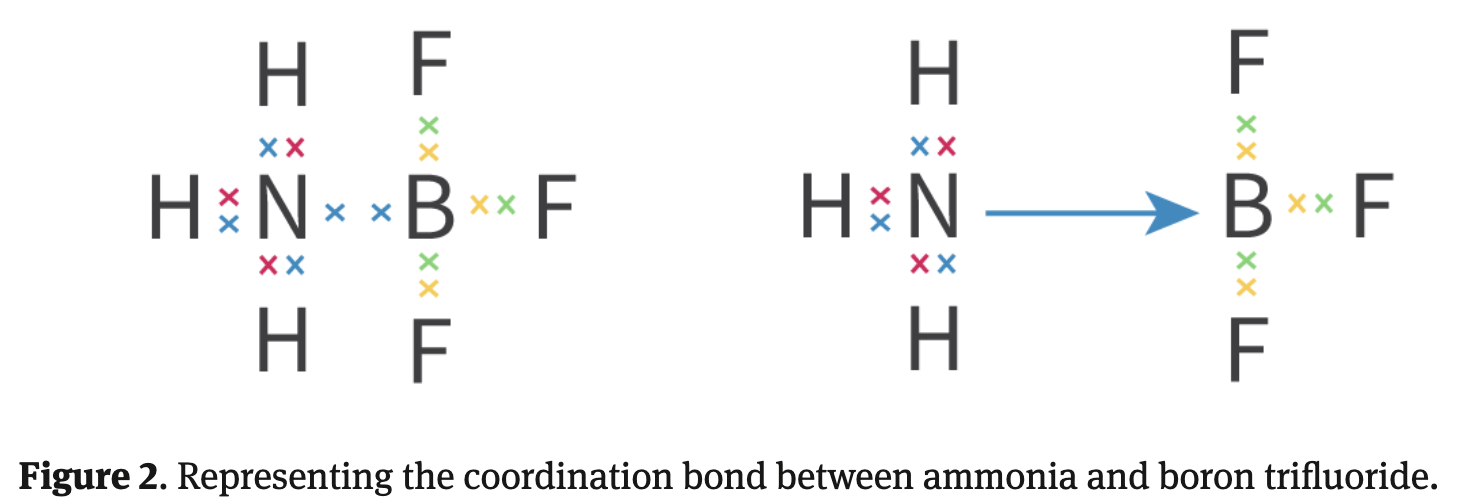
Central atom
A in the AXE
Bonding pairs of electrons
X in AXE
Non-bonding electron pairs
E in AXE
180° linear
AX
180° linear
AX2

180° linear
AXE
120° trigonal planar
AX3
<120° angular/bent
AX2E
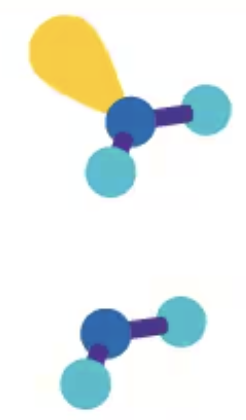
180° linear
AXE2

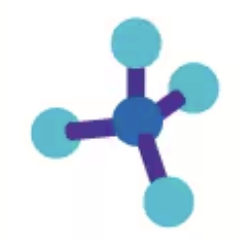
109.5° tetrahedral
AX4
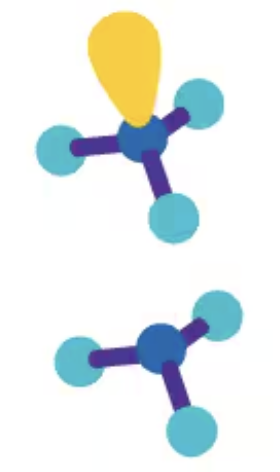
<109.5° triangular pyramidal
AX3E
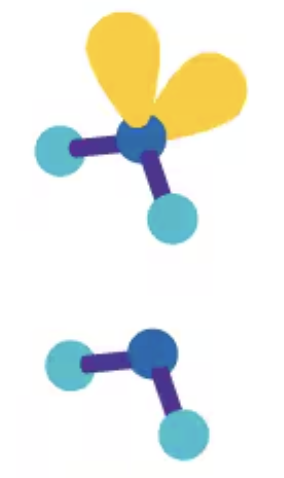
«109.5 angular/bent
AX2E2
180° linear
AXE3
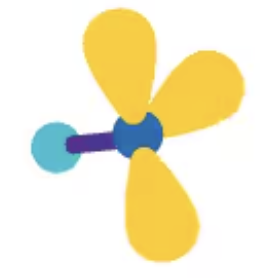
trigonal bipyramidal
AX5
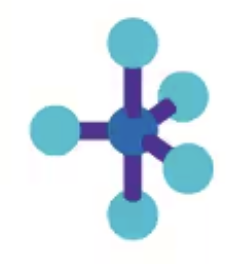
Seesaw
AX4E
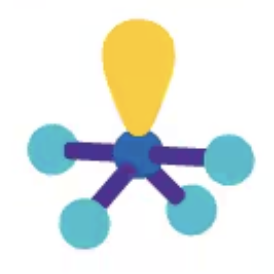
T-shaped
AX3E2
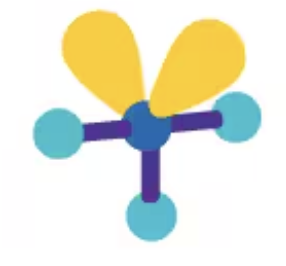
Linear
AX2E3

Octahedral
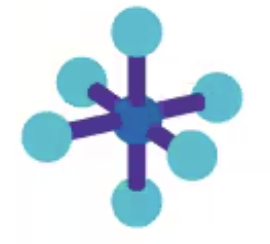
AX6
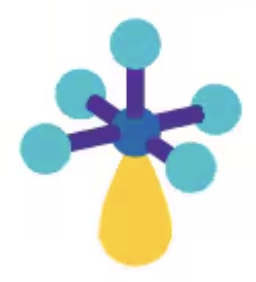
Square pyramidal
AX5E
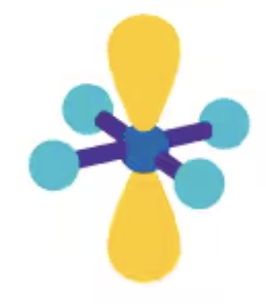
Square planar
AX4E2
Electron domains
The region in which bonding and non-bonding pairs of electrons are most likely to be found. Non-bonding pairs, single bonds, double bonds and triple bonds each count as one electron domain.
VSEPR Theory: Valence Shell Electron Pair Repulsion Theory: Linking of electron domains, bond angles, and resulting electron domain geometry. Used to predict the electron domain geometry of the molecule.
Based on the ideas that:
Electron pairs, referred to as electron domains in VSEPR theory, repel each other. Note that it is focused only on the valence electrons of a central atom; electrons in inner-shells are not considered.
An electron domain can be either a single bond, a double bond, a triple bond or a non-bonded pair of electrons. Multiple bonds count as one domain.
VSEPR Theory: Valence Shell Electron Pair Repulsion Theory: Linking of ____ domains, bond _____, and resulting electron _____ geometry. Used to predict the electron domain geometry of the molecule.
Based on the ideas that:
Electron pairs, referred to as electron domains in VSEPR theory, _____ each other. Note that it is focused only on the _____ electrons of a central atom; electrons in ____-shells are not considered.
An electron domain can be either a single bond, a double bond, a triple bond or a non-bonded pair of electrons. Multiple bonds count as one domain.
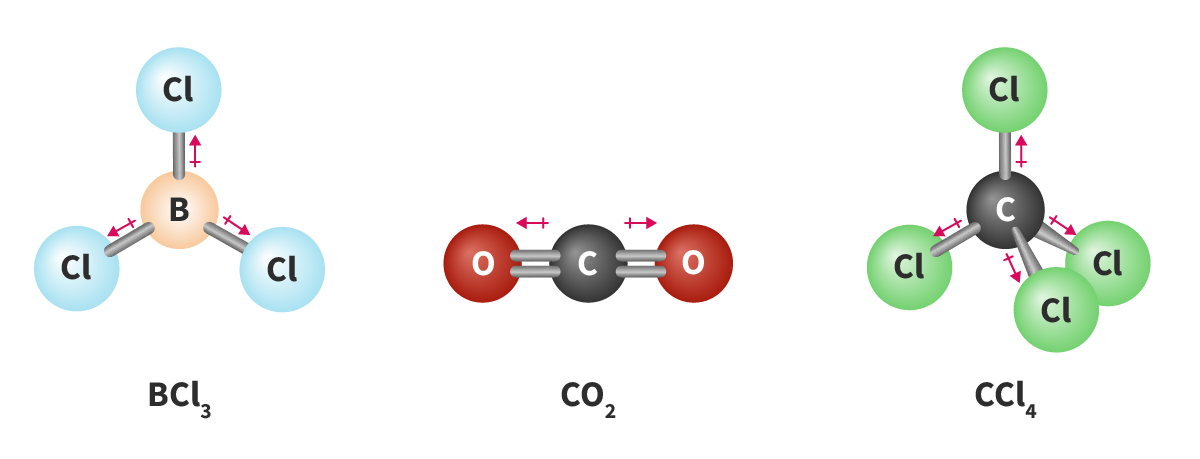
Bond polarity:
How to check polarity for covalent compounds:
Check bonds: Polar or non-polar?
If polar, check structure and equal/unequal pull
If nonpolar, always nonpolar
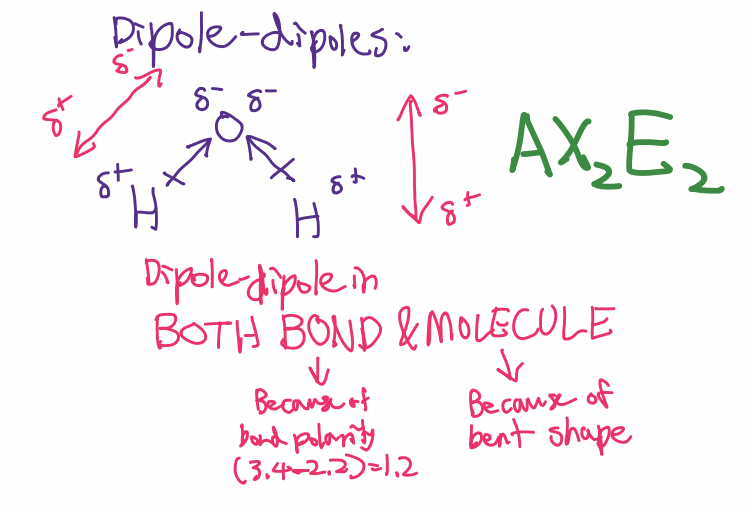
How to check ______ for covalent compounds:
Check _____: Polar or non-polar?
If polar, check _____ and equal/unequal ___
If nonpolar, always ____
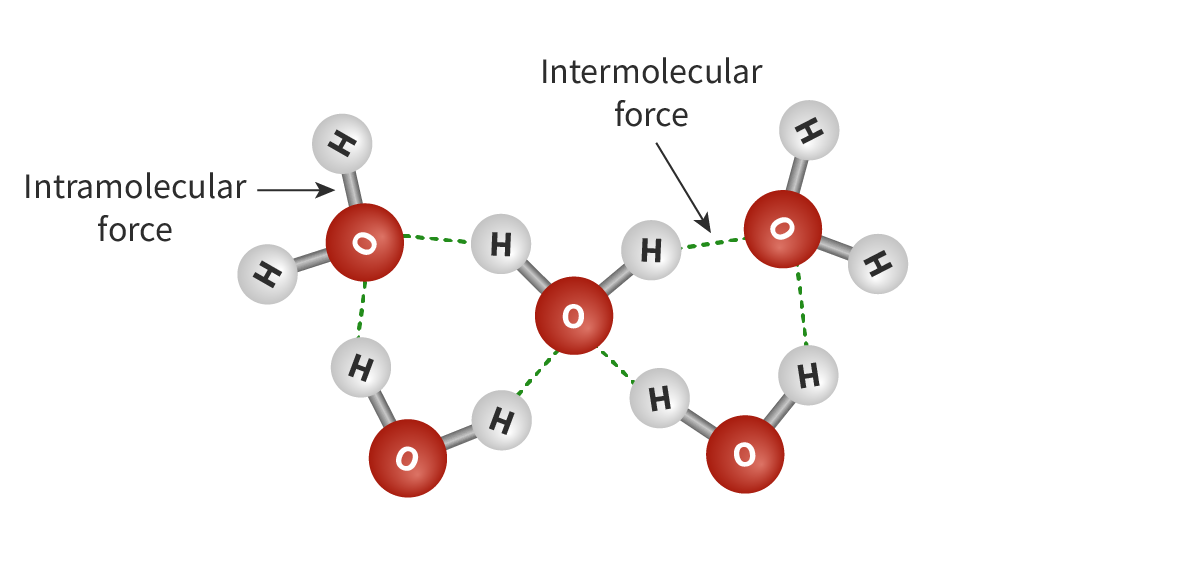
Intramolecular forces
The covalent bonds between atoms within a molecule. Example: Ionic, covalent, metallic bonds
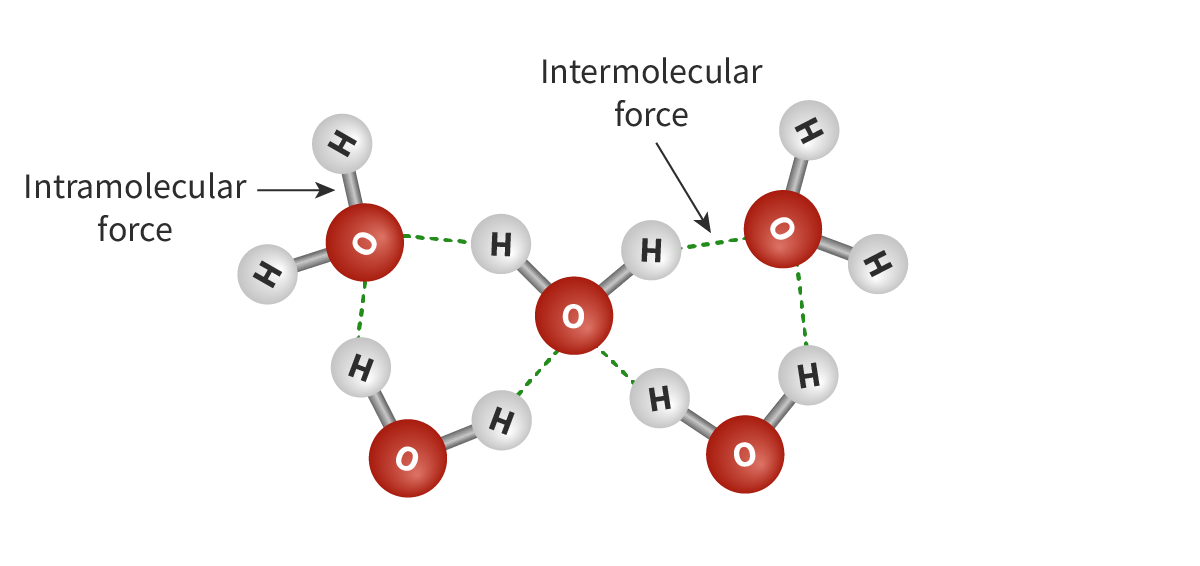
Intermolecular forces
Attractive (or repulsive) forces that exist between the molecules of a substance.
Bromine
Example of a molecule with both intermolecular and intramolecular forces
Van Der Waals forces
A general term for weak intermolecular forces that exist between atoms and molecules
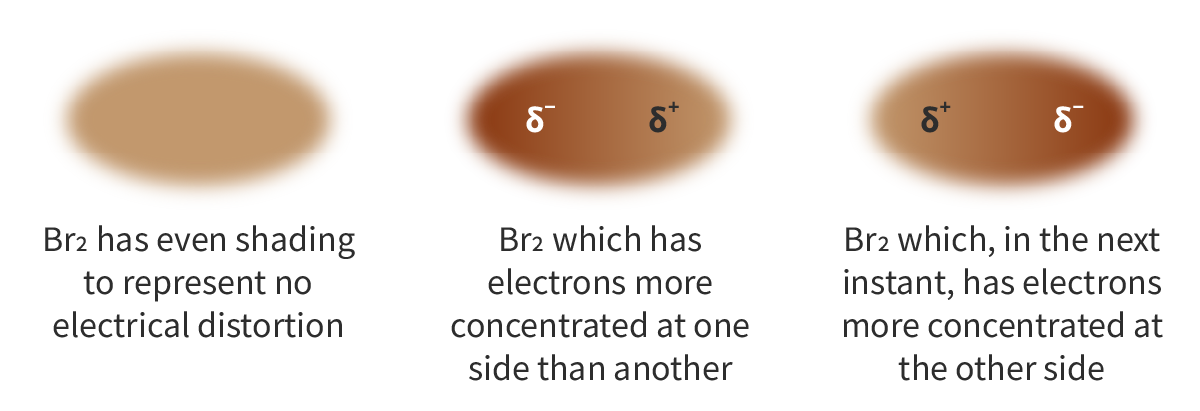
London-dispersion forces: The random, continuous movement of electrons can create asymmetrical electron distribution which causes attraction between neighbouring atoms or molecules
Electrons are continuously moving, thus, though on average, there is no net dipole between symmetrical molecules, there is 1 moment where electrons randomly end up on one side, making a partial negative, followed by another moment where electrons end up on the other.
Thus, these temporary dipoles cause attraction between neighbouring molecules that both experience the asymmetry in electron distribution
LDFs exist in all molecules, regardless of the level of electronegativity difference, though weak.
London-dispersion forces: The _____, continuous movement of electrons can create __________ electron distribution which causes attraction between neighbouring atoms or molecules
Electrons are continuously moving, thus, though on average, there is no ___ _____ between symmetrical molecules, there is 1 moment where electrons randomly end up on one side, making a partial negative, followed by another moment where electrons end up on the other.
Thus, these _____ dipoles cause attraction between neighbouring molecules that both experience the asymmetry in electron distribution
LDFs exist in _____ molecules, regardless of the level of electronegativity difference, though weak.
Dipole-induced dipole: A weak intermolecular force that occurs when a polar molecule gets close to an atom or non-polar molecule and induces a dipole by disrupting the arrangement of its electrons
Because of the powerful dipoles of a polar molecule, even nonpolar molecules form temporary dipoles (because electrons move)
LARGER molecules are MORE susceptible to form dipole-induced dipoles, as they have larger electron clouds = Electrons not held as tightly by nucleus = Greater scope for distortion
Dipole-induced dipole: A ____ intermolecular force that occurs when a ___ molecule gets close to an atom or non-polar molecule and ____ a dipole by disrupting the _______ of its _______
Because of the powerful dipoles of a polar molecule, even nonpolar molecules form _______ dipoles (because electrons move)
LARGER molecules are _____ susceptible to form dipole-induced dipoles, as they have ______ electron clouds = Electrons not held as tightly by nucleus = Greater scope for _____
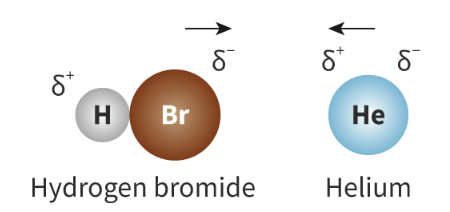
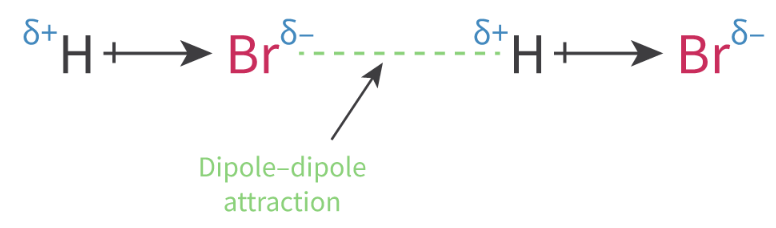
Dipole-dipole: A intermolecular force between two polar molecules that have permanent dipoles. Attractive forces exist between the positive end of one polar molecule and the negative end of another polar molecule
Asymmetrical molecules = polar
Regions of permanent partial charges form
Only exists between 2 ASYMETTRICAL MOLECULES that each have a PERMANENT DIPOLE
Dipole-dipole: A intermolecular force between two _____ molecules that have ________ dipoles. Attractive forces exist between the _____ end of one polar molecule and the negative end of another polar molecule
Asymmetrical molecules = polar
Regions of permanent partial charges form
Only exists between 2 ASYMETTRICAL MOLECULES that each have a PERMANENT DIPOLE
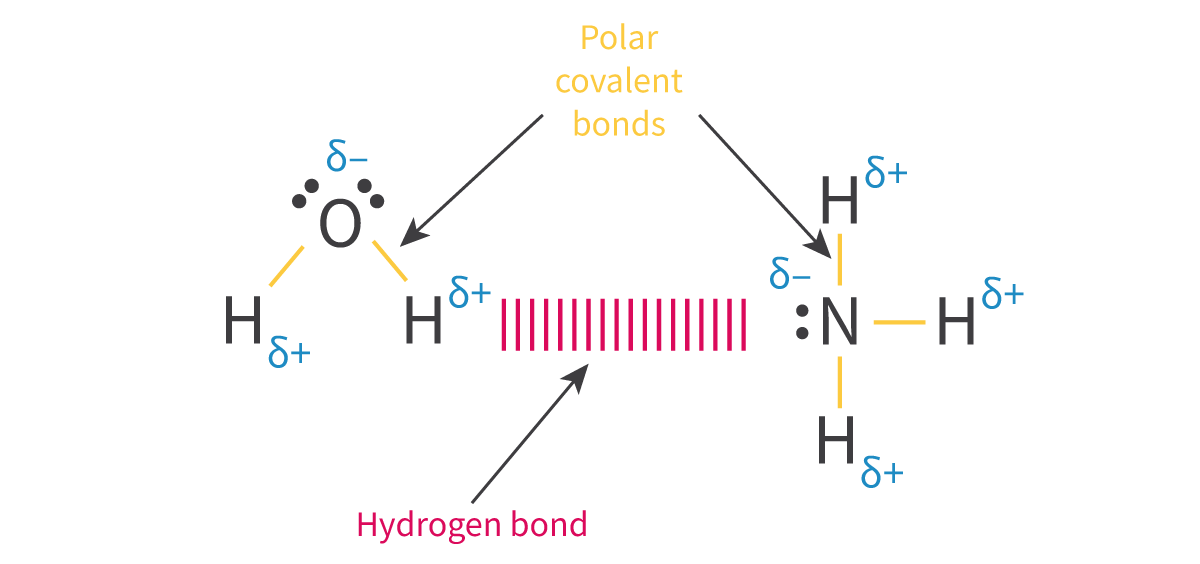
Hydrogen bonding: The intermolecular attraction between two molecules which both contain a hydrogen bonded to a highly electronegative element such as oxygen, fluorine or nitrogen
Requirements:
Hydrogen bonded to a FON element
FON element must have a non-bonding pair of electrons
Very significant electronegativity difference between H and FON --> Hydrogen can have strong ẟ+, which is only amplified by the fact that hydrogen has no inner electrons to shield the nucleus's pull --> ẟ+ hydrogen very attracted to non-bonding pairs of electrons on neighbouring molecules
Hydrogen bonding: The intermolecular attraction between two molecules which both contain a ______ bonded to a highly ________ element such as oxygen, fluorine or nitrogen
Requirements:
Hydrogen bonded to a _____ element
____ element must have a ___-_____ pair of electrons
Very significant electronegativity _______ between H and FON --> Hydrogen can have strong ẟ+, which is only amplified by the fact that hydrogen has no inner electrons to shield the nucleus's pull --> ẟ+ hydrogen very attracted to ___-bonding pairs of electrons on neighbouring molecules
type of force and molecular size
2 factors affecting strength of intermolecular forces:
electrical conductivty, volatility, solubility
3 properties of covalent molecules:
Properties of covalent molecules in general:
Electrical conductivity and melting and boiling points
Molecules = Not electrically conductive (don't have ions or delocalized electrons like in ionic compounds or carbon allotropes)
Don't have same high melting/boiling points as ionic/metallic compounds (as comparatively weak intermolecular forces require less energy to break)
Volatility:
Depends on the type of intermolecular force that exists between the molecules of the substance
Stronger intermolecular force = Low volatilities + high boiling points
HOWEVER, covalent network structures have high boiling points because of strong covalent bonds)
Solubility:
Soluble molecules --> form homogenous mixtures with uniform properties
General guideline: "like dissolves like" = Polar molecules will dissolve in polar solvents, and vice versa
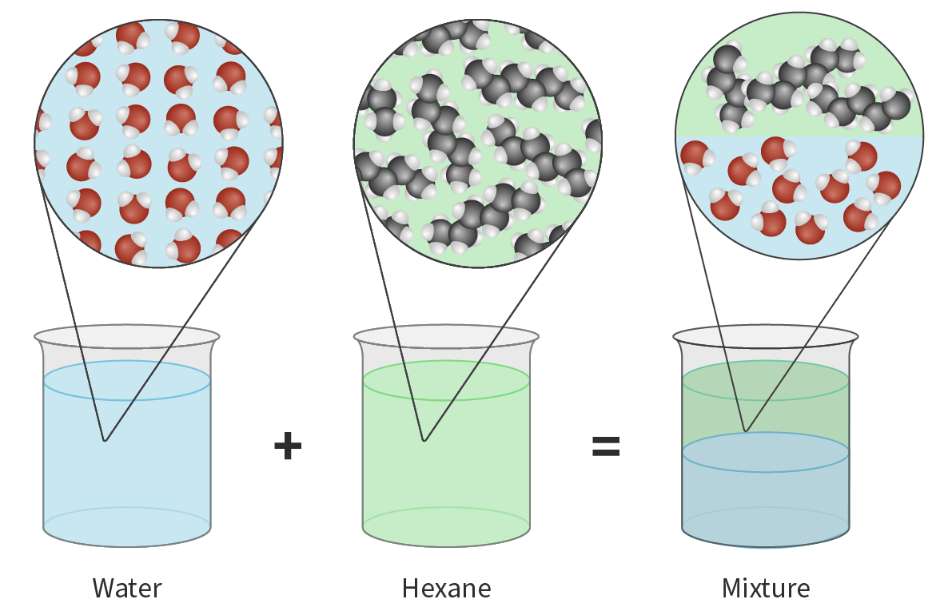
Example: Iodine molecules dissolve in nonpolar solvents (like hexane) because of iodine's ability to interact with hexane via LDFs --> Form homogenous mixture with hexane, but NOT strong enough to overcome hydrogen bonds with polar solvents (like water) --> Form heterogenous mixtures
Solubility of some polar molecules will decrease as molecule size increases (as polar part of the molecule becomes relatively small compared to the size of the overall molecule)
Ex. Hexanol less soluble in water than ethanol
Covalent network structures = insoluble in both polar and nonpolar solvents
Properties of covalent molecules in general:
_______ conductivity and melting and boiling points
Molecules = Not electrically conductive (don't have ions or _______ electrons like in ionic compounds or carbon allotropes)
Don't have same high melting/boiling points as ionic/metallic compounds (as comparatively ______ intermolecular forces require less energy to break)
Volatility:
Depends on the type of intermolecular force that exists between the molecules of the substance
_______ intermolecular force = Low volatilities + high boiling points
HOWEVER, _______ network structures have high boiling points because of strong covalent bonds)
Solubility:
Soluble molecules --> form _______ mixtures with uniform properties
General guideline: "like dissolves like" = Polar molecules will dissolve in polar solvents, and vice versa
Example: Iodine molecules dissolve in nonpolar solvents (like hexane) because of iodine's ability to interact with hexane via LDFs --> Form homogenous mixture with hexane, but NOT strong enough to overcome hydrogen bonds with polar solvents (like water) --> Form heterogenous mixtures
Solubility of some polar molecules will ________ as molecule size increases (as polar part of the molecule becomes relatively small compared to the size of the overall molecule)
Ex. Hexanol less soluble in water than ethanol
Covalent network structures = ______ in both polar and nonpolar solvents
Allotrope
Alternative forms of an elemental substance. For example, diamond and graphite are allotropes of carbon.
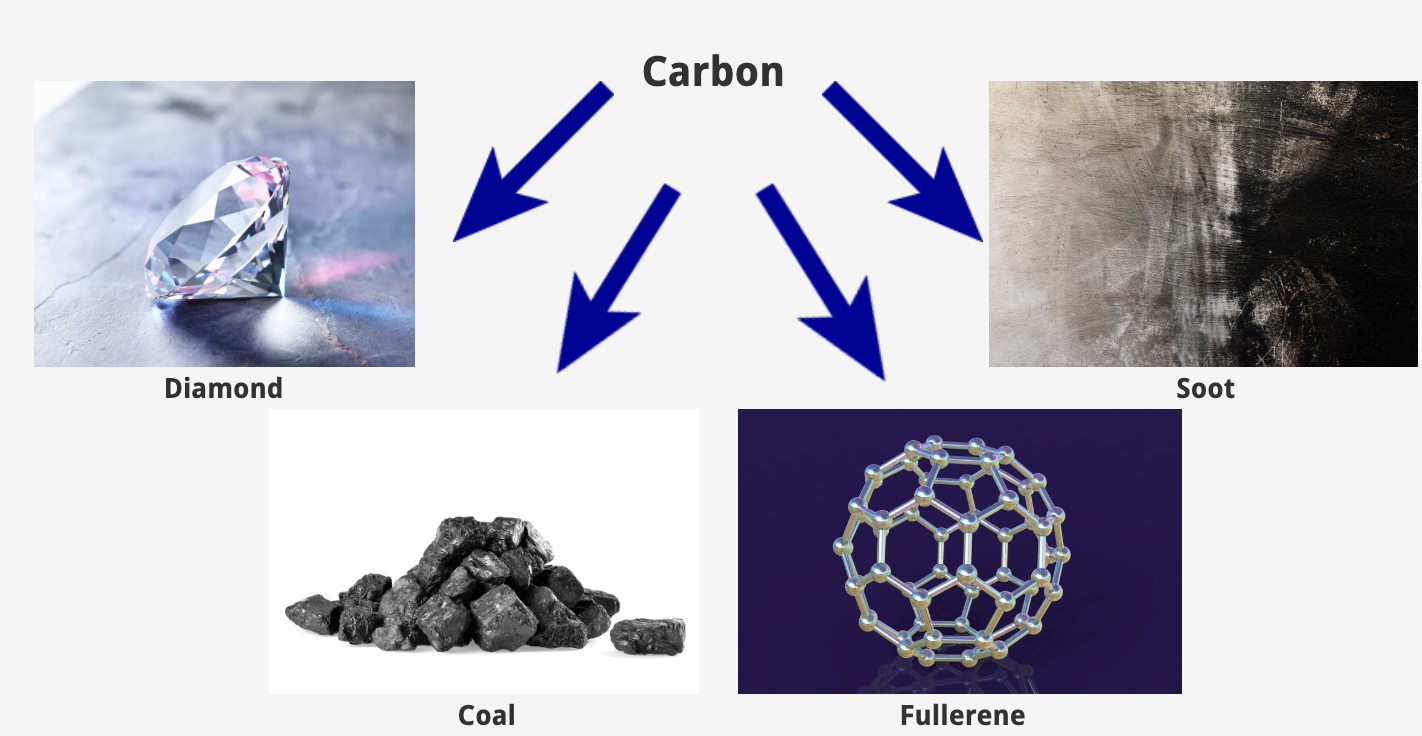
fullerene, diamond, graphite, graphene
4 allotropes of carbon are:
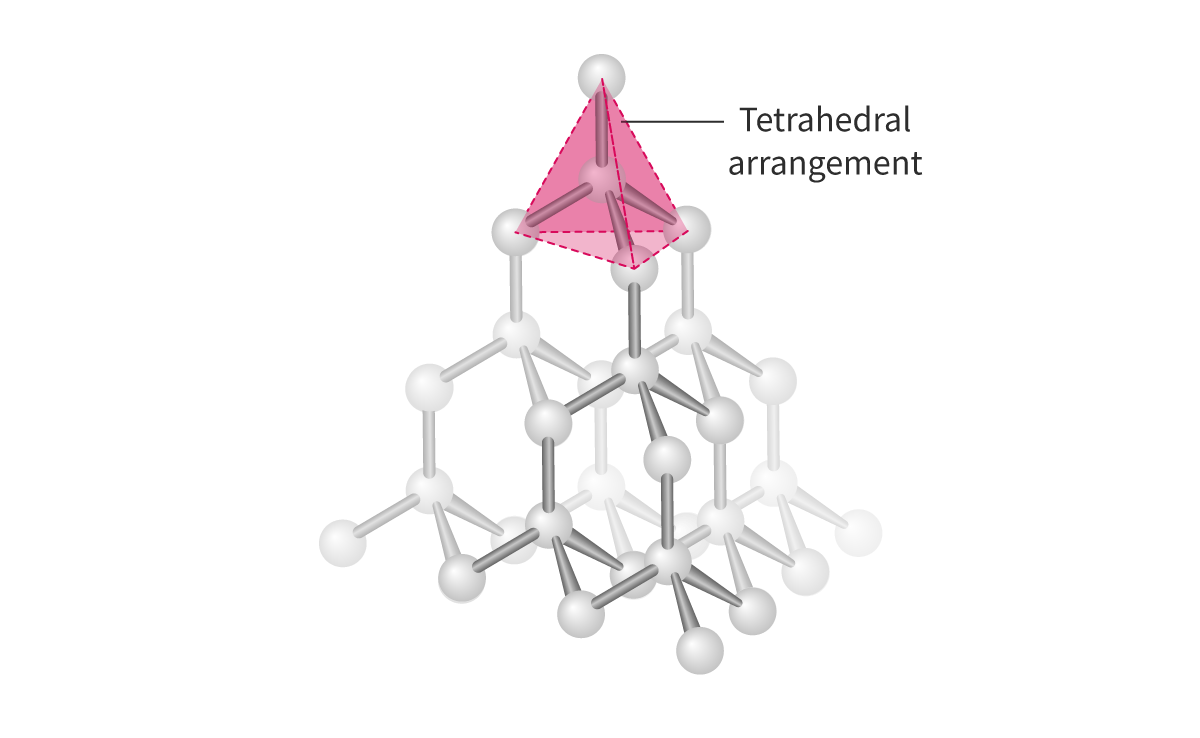
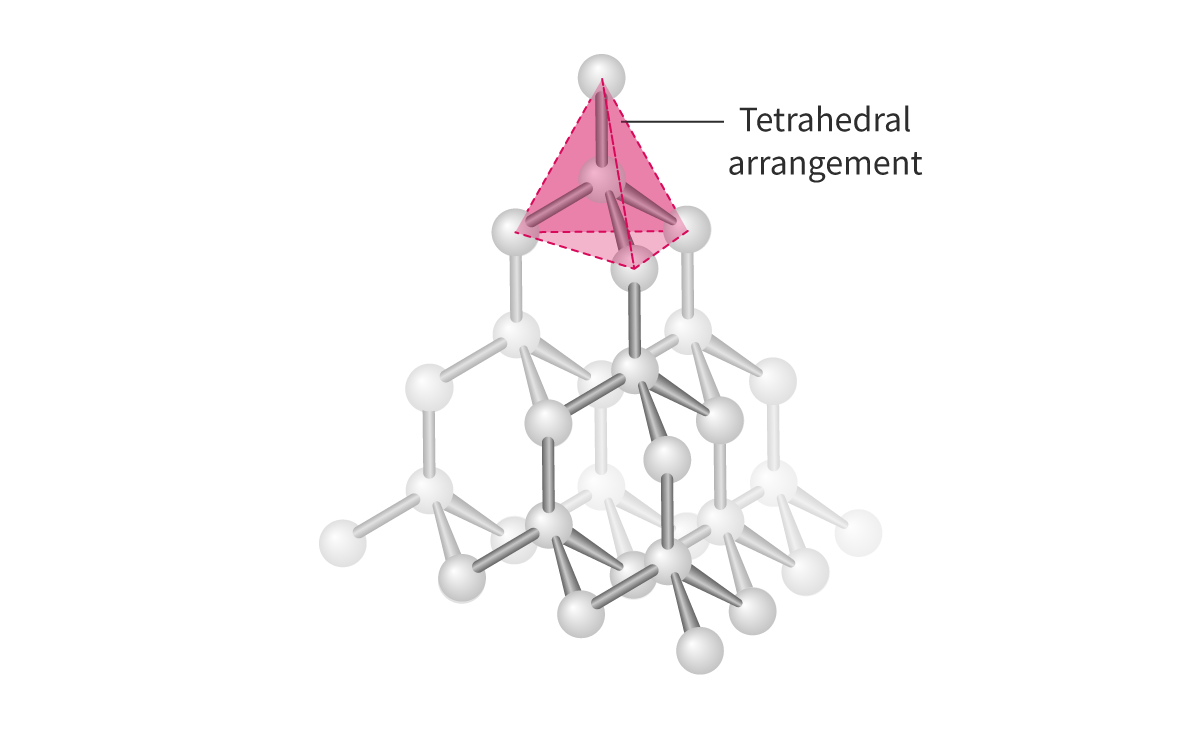
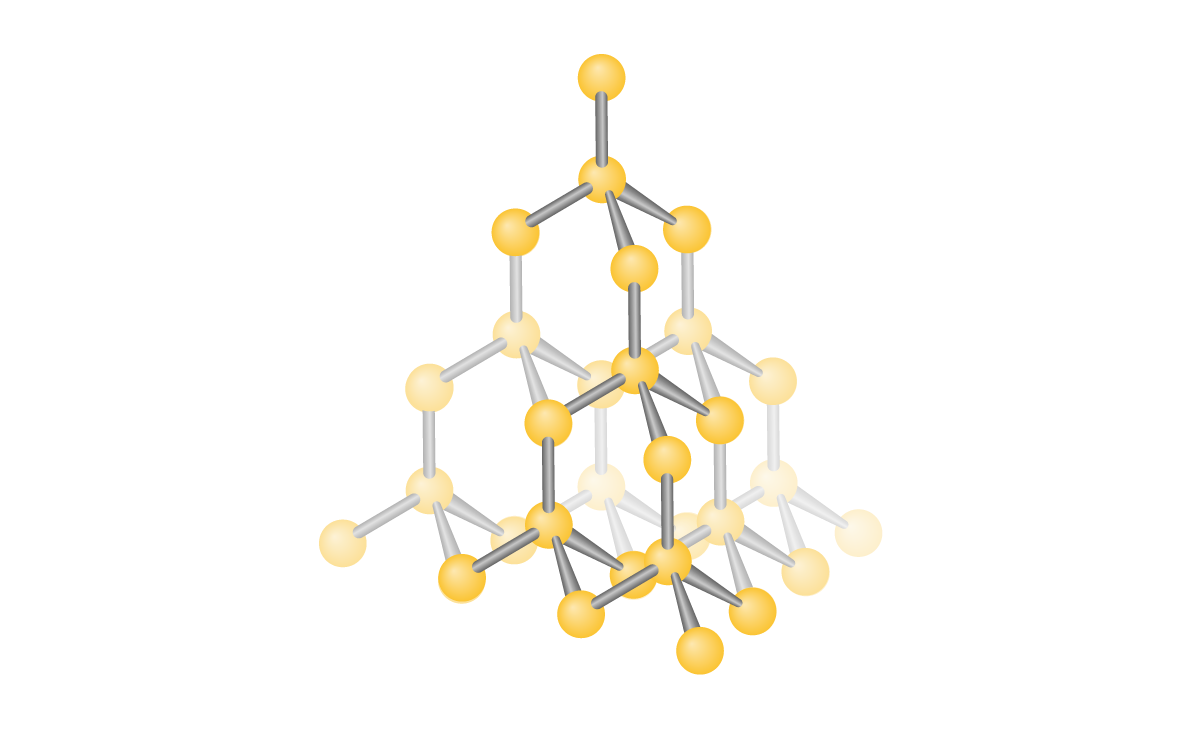
Diamond:
- Diamond is a covalent network structure made up of carbon atoms connected to each other, which theoretically can continue infinitely. Each carbon in diamond is covalently bonded to four other carbons
- Tetrahedral
- 109.5°
- Strong structure
- 0 pi, 4 sigma bond
- Sp3
- NO DELOCALIZED electrons = cannot conduct electricity
Diamond:
- Diamond is a covalent network structure made up of carbon atoms connected to each other, which theoretically can continue infinitely. Each carbon in diamond is covalently bonded to four other carbons
- ________
- ____°
- Strong structure
- ___ pi, ____ sigma bond
- Sp___
- NO DELOCALIZED electrons = ______ conduct electricity
Graphite:
- Graphite contains carbon atoms which are joined to three other carbons
The stacked layers in graphite are held together by relatively weak
London dispersion forces
- As these London dispersion forces holding layers of carbons together are fairly weak, this makes them easy to separate. This is why graphite is quite soft and slippery: the layers are easy to separate. This makes graphite ideal for use in pencils, each layer can slide off onto the page with very little force applied. This is unlike diamond. The ability of the layers in graphite structures to slide over each other also make it ideal for use as a ‘dry’ lubricant. This is particularly useful in situations where heat might be present, which might otherwise cause an oil-based lubricant to ignite.
- Trigonal planar
- 120°
- 1 pi, 3 sigma bond
Bond order: 1.5
Graphite:
- Graphite contains carbon atoms which are joined to three other carbons
The stacked layers in graphite are held together by relatively weak
London dispersion forces
- As these London dispersion forces holding layers of carbons together are fairly weak, this makes them easy to separate. This is why graphite is quite soft and slippery: the layers are easy to separate. This makes graphite ideal for use in pencils, each layer can slide off onto the page with very little force applied. This is unlike diamond. The ability of the layers in graphite structures to slide over each other also make it ideal for use as a ‘dry’ lubricant. This is particularly useful in situations where heat might be present, which might otherwise cause an oil-based lubricant to ignite.
- ______ _______
- ____°
- ___ pi, ___ sigma bond
Bond order: 1.5
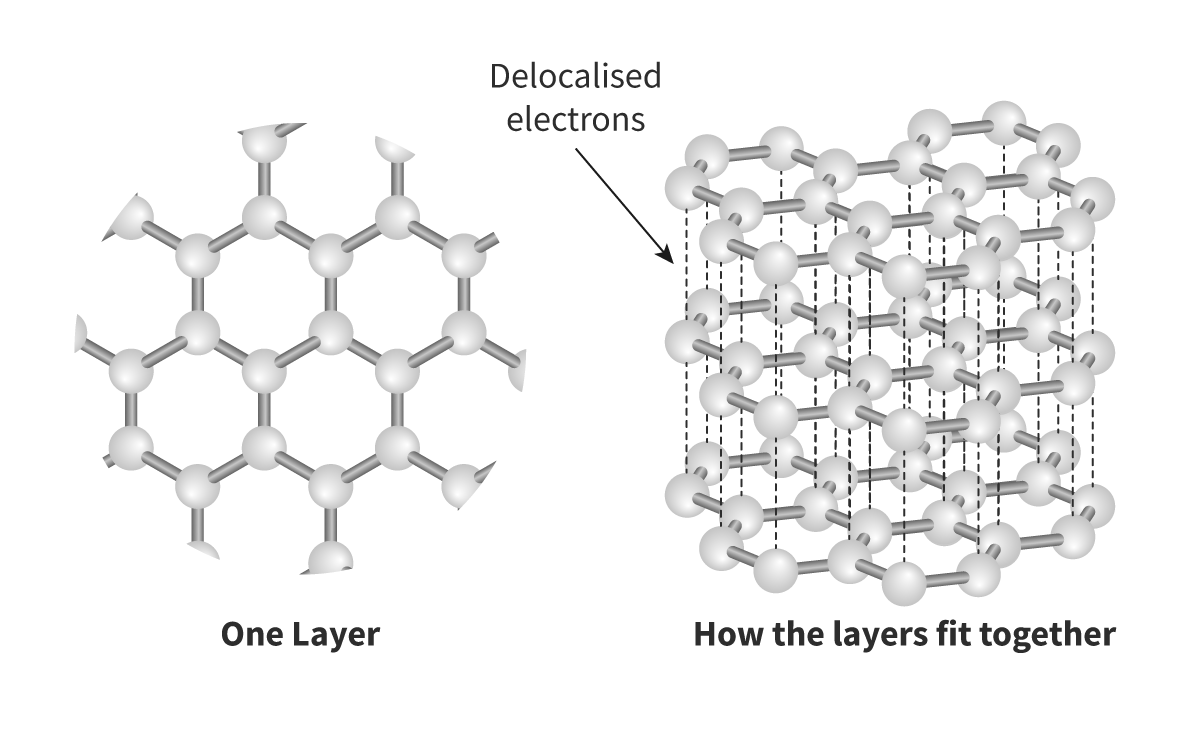
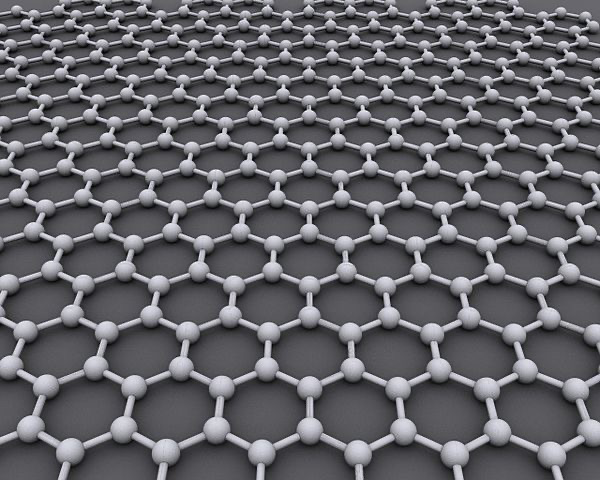
Graphene:
- Graphene is composed of just one layer of graphite and is said to be the strongest, lightest and thinnest material known to humans. It is so thin, just one carbon atom thick, that it can technically be referred to as being two-dimensional. Whereas both diamond and graphite occur naturally, graphene is a synthetic carbon allotrope.Each carbon in graphene is bonded to three other carbons with no non-bonding pairs
- Graphene is the strongest material to ever exist. It is 100 times stronger than steel and also has very high thermal conductivity
- Electrons can move all around the carbons —> Delocalized —> Can conduct electricity
- Sea of electrons that can exist above and below, because graphite is a flat sheet
- Sp2
Graphene:
- Graphene is composed of just ____ layer of graphite and is said to be the strongest, lightest and thinnest material known to humans. It is so thin, just one carbon atom thick, that it can technically be referred to as being two-dimensional. Whereas both diamond and graphite occur naturally, graphene is a synthetic carbon allotrope.Each carbon in graphene is bonded to three other carbons with no non-bonding pairs
- Graphene is the _______ material to ever exist. It is 100 times stronger than steel and also has very high ______ conductivity
- Electrons can move all around the carbons —> Delocalized —> Can conduct electricity
- Sea of electrons that can exist above and below, because graphite is a flat sheet
- Sp____
Fullerene:
- Trigonal planar geometry
- Bond angle 120° (but, because spherical shape, a bit less)
- Mix of both 20 hexagonal and 12 pentagonal rings
Have Delocalized electrons, but they’re limited - they can only move around each fullerene, and not move between 2 separate fullerenes = limited conductivity, can’t move in linear path as with graphite and graphene
Fullerene:
- ______ _____ geometry
- Bond angle ____° (but, because spherical shape, a bit less)
- Mix of both 20 hexagonal and 12 pentagonal rings
Have ______ electrons, but they’re _____ - they can only move around each fullerene, and not move between 2 separate fullerenes = limited ______, can’t move in linear path as with graphite and graphene
Silicon:
- Contains silicon atom that’s covalently bonded to 4 other silicons
Silicon:
- Contains silicon atom that’s ________ bonded to ____ other silicons
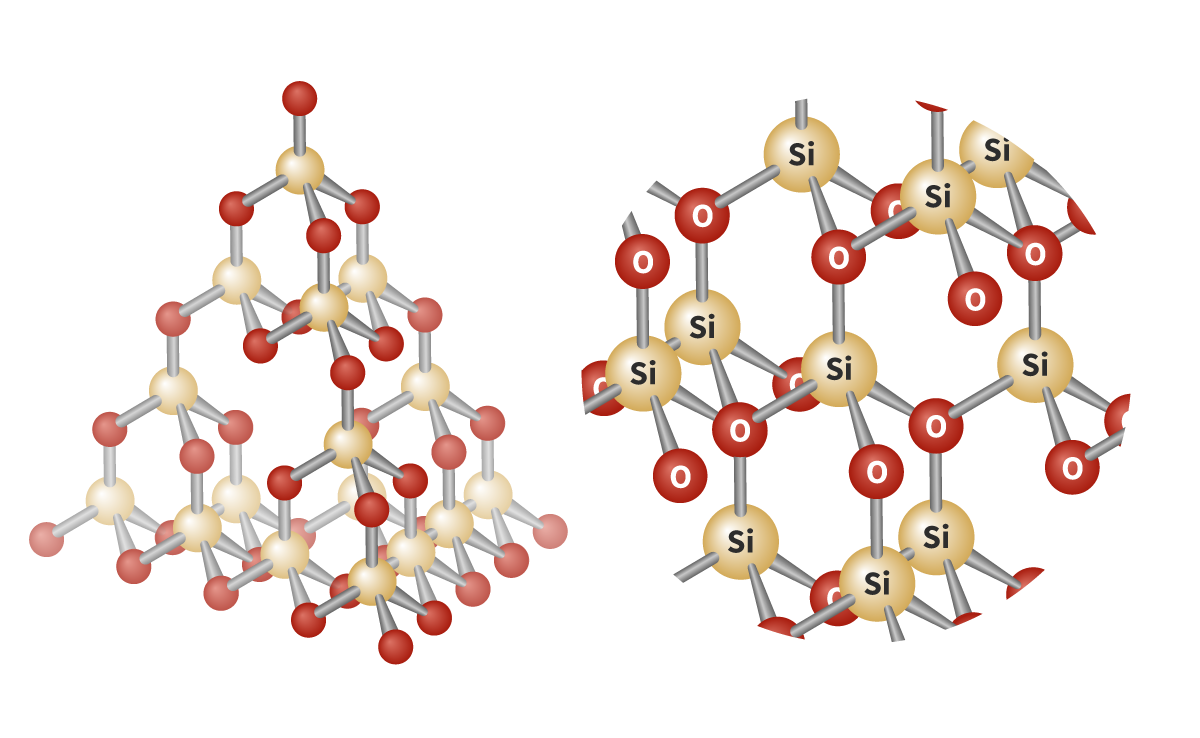
Silicon dioxide:
- Compound that's also a covalent network structure
- Covalent network structure:
- SiO2 Empirical formula
- Silicon bonded to 4 oxygen atoms
- Tetrahedral geometry
- Bond angle 109.5°
- High melting and boiling point
Silicon dioxide:
- Compound that's also a covalent ______ structure
- Covalent network structure:
- SiO2 Empirical formula
- Silicon bonded to ___ oxygen atoms
- _______ geometry
- Bond angle ______°
- _____ melting and boiling point
Bond Order
Collective term used to describe the properties of single, double and triple bonds, includes bond enthalpy and bond length
Resonance Energy/Stabilization Energy
The additional stability that a resonance hybrid molecule has due to the delocalised electrons. Also known as stabilisation energy.
Addition reaction
A reaction in which an atom or molecule is added to another molecule which contains a double or triple bond
Substitution reaction
A reaction that involves an atom or group of atoms of a reactant molecule replacing an atom or group of atoms which are attached to a carbon atom in the other reactant molecule.
3 reasons why Benzene is special:
C-C bonds are all the same length, with the same bond angle
Benzene is more stable than expected due to resonance energy
Benzene is reluctant to undergo addition reactions and prefers substitution reactions
3 reasons why Benzene is special:
_____ bonds are all the same length, with the same bond ___
Benzene is more _____ than expected due to _______ energy
Benzene is reluctant to undergo _____ reactions and prefers ______ reactions
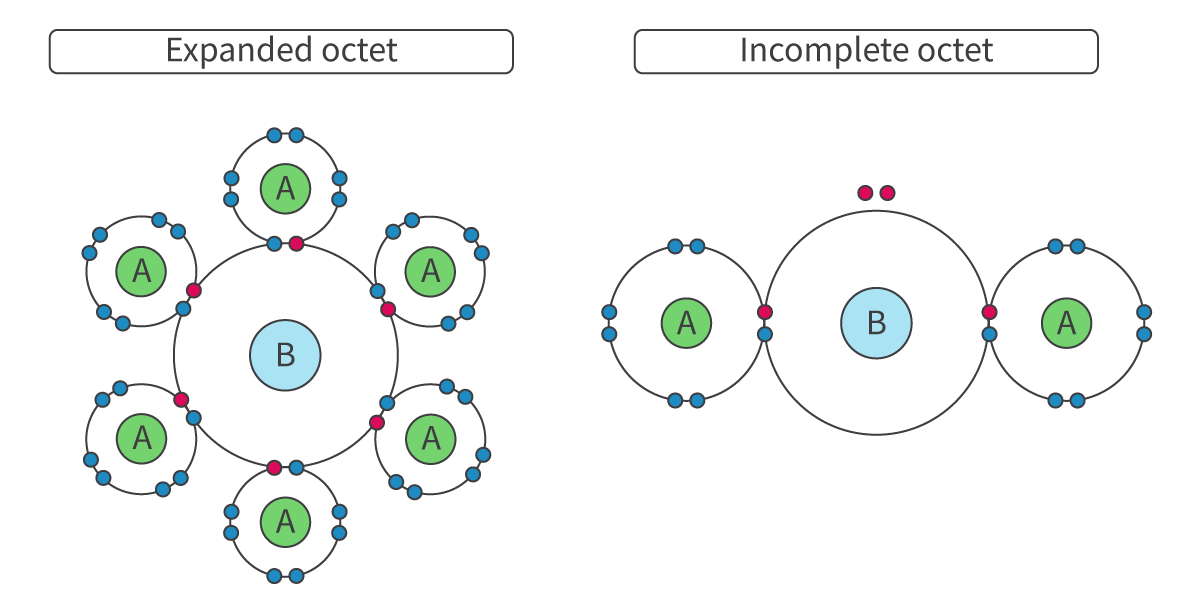
Expanded Octet
The ability of a central atom from period 3 or below to achieve stability with more than 8 electrons in its outer shell. For example, Xe, P or S
Only possible for Period 3 onwards
WHY? Have empty 3d-orbitals (close in energy to 3p-orbitals) = Possible to promote electrons from 3p to 3d orbitals if needed --> Allow additional electron pairs to form
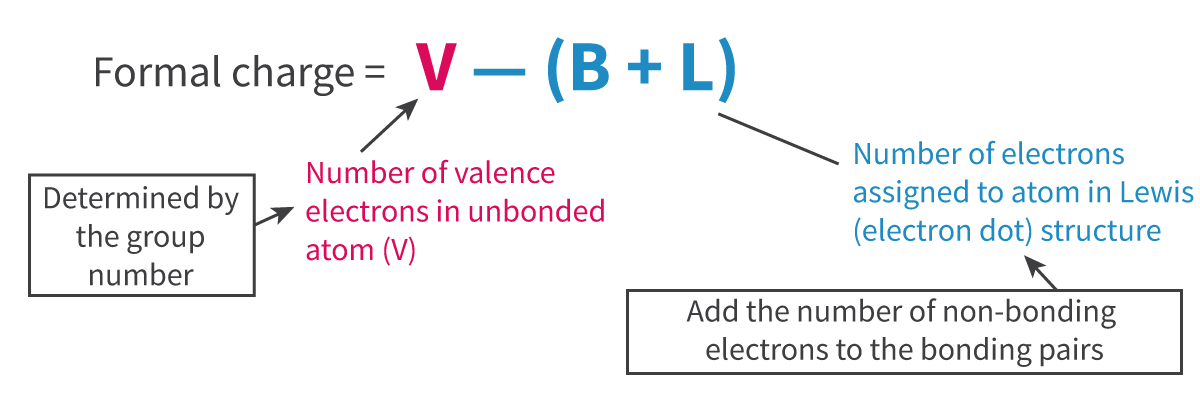
valence - (nonbonding electrons + bonding pairs)
Formal charge formula:
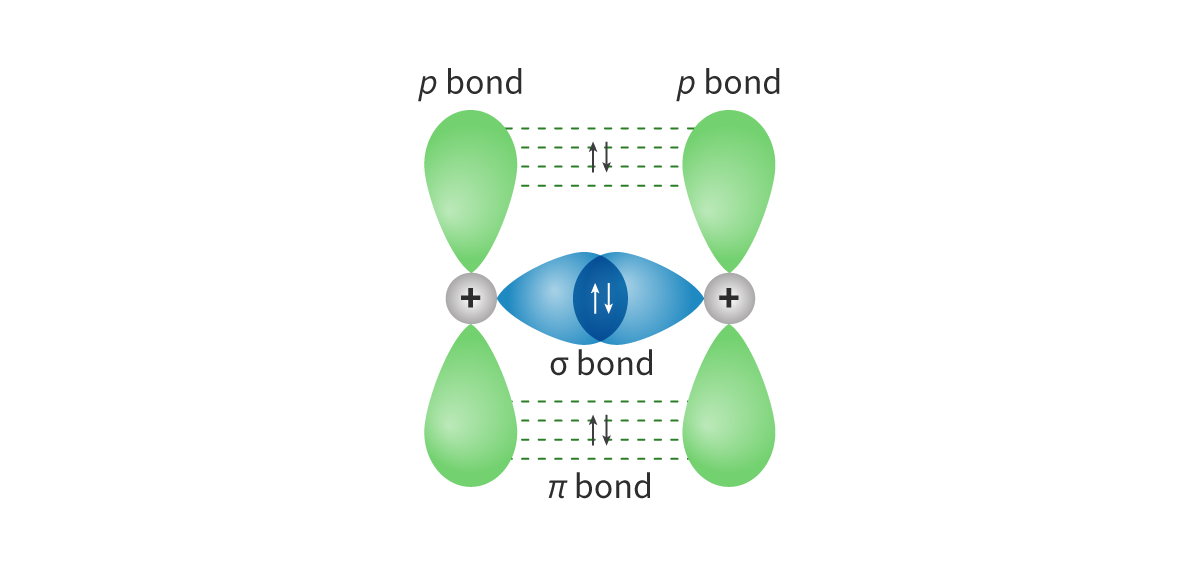
Sigma bond: A bond formed when atomic orbitals overlap head-on(axial overlap) and the electron density is concentrated along the bond axis, where bond rotation is possible
Sigma bond: A bond formed when atomic _____ overlap ____-on(axial overlap) and the electron _____ is concentrated along the bond ___, where bond rotation is ____
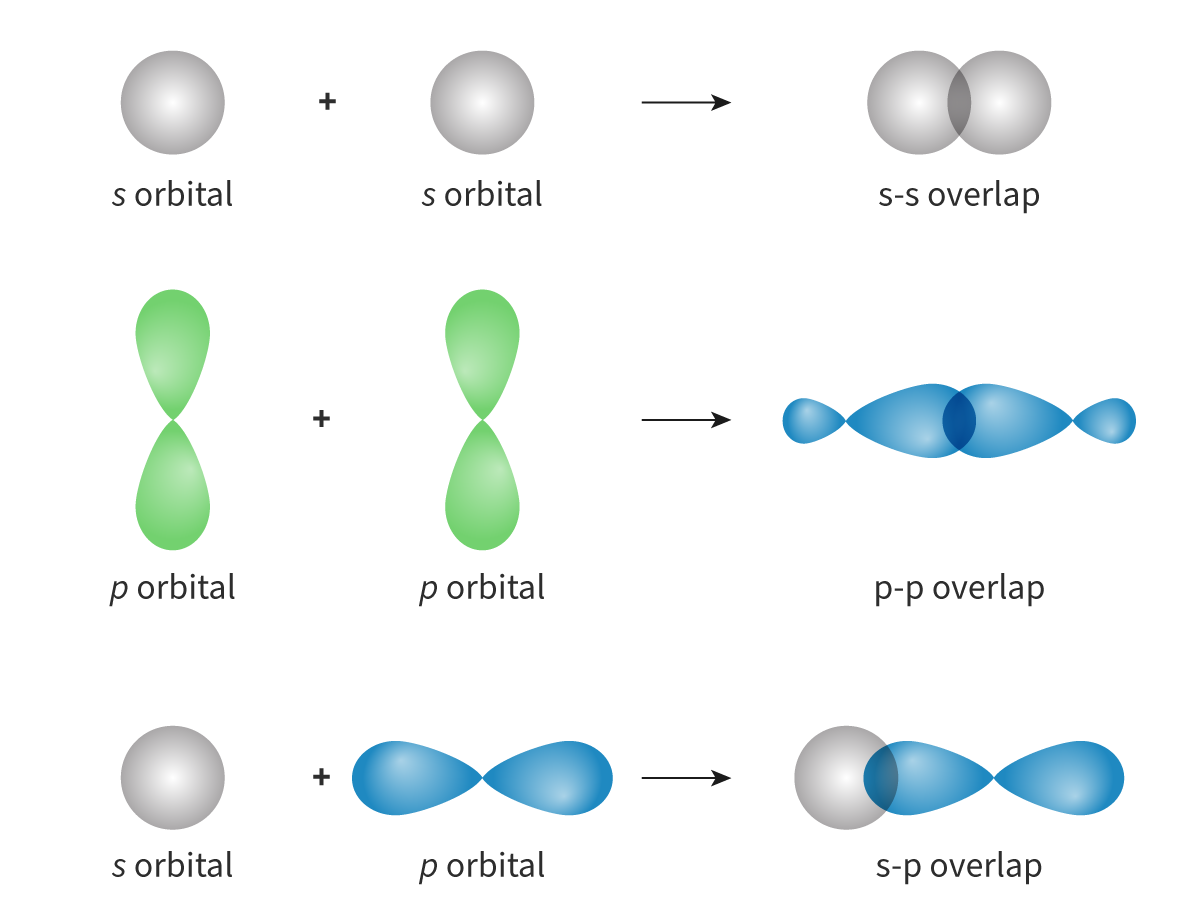
- Pi bond: A bond formed when one atomic orbital overlaps sideways with another atomic orbital, with electron density concentrated on opposite sides of the bond axis
Bond rotation not possible (2 regions of electron density located above and below the plane of the internuclear axis)
- Pi bond: A bond formed when one atomic ____ overlaps ______ with another atomic orbital, with electron ____ concentrated on _____ sides of the bond axis
Bond rotation ___ possible (2 regions of electron _____ located above and below the plane of the _______ axis)
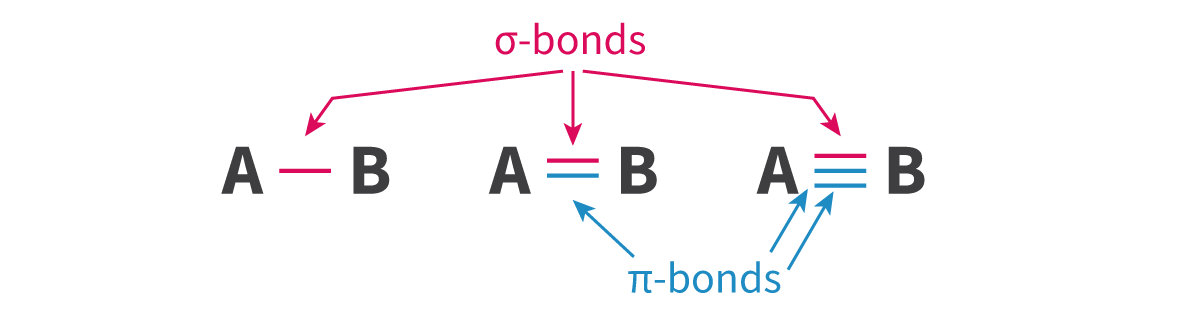
Pi < Sigma
Strength of sigma and pi bonds
○ Sigma bond has stronger attraction between electron pair and positive nuclei, as theres only 1 electron-dense region on the inter-nuclear axis located close to the nuclei
○ In a pi bond, the electron density is spread across 2 regions (above + below the internuclear axis) --> therefore further from the positive charge of the nucleus = weaker
○ Pi bond easier to break than sigma bonds = Double and triple bonds are more reactive than single bonds
○ Sigma bond has _____ attraction between electron pair and positive nuclei, as theres only 1 electron-dense region on the inter-nuclear axis located close to the nuclei
○ In a pi bond, the electron density is _____ across ___ regions (above + below the internuclear axis) --> therefore further from the _____ charge of the nucleus = weaker
○ Pi bond easier to break than sigma bonds = Double and triple bonds are more ______ than single bonds
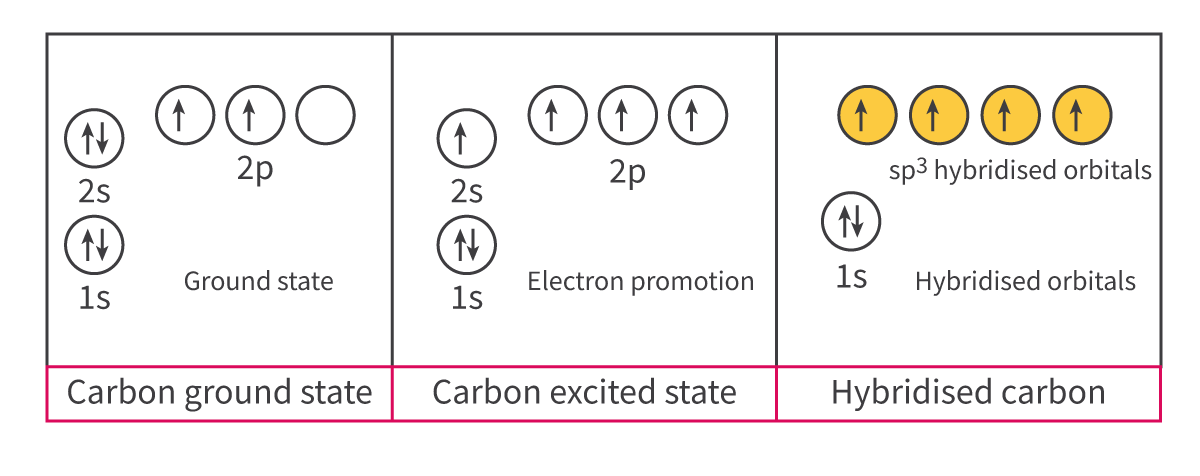
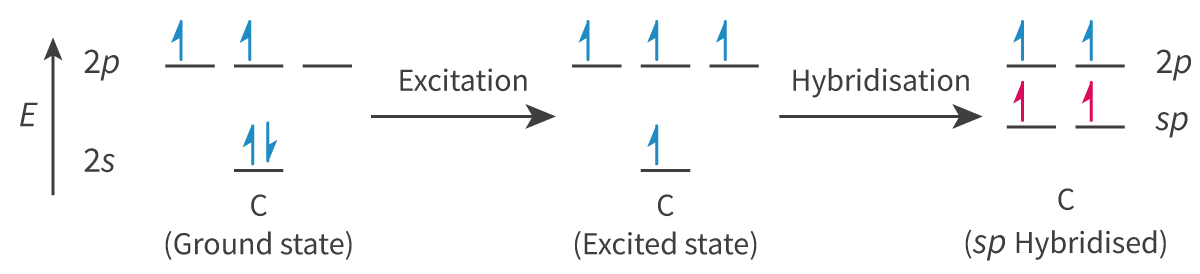
Hybridization: The process by which atomic orbitals within an atom mix to produce hybrid orbitals of intermediate energy
Hybridization: The process by which ____ orbitals within an atom ___ to produce ____ orbitals of ______ energy
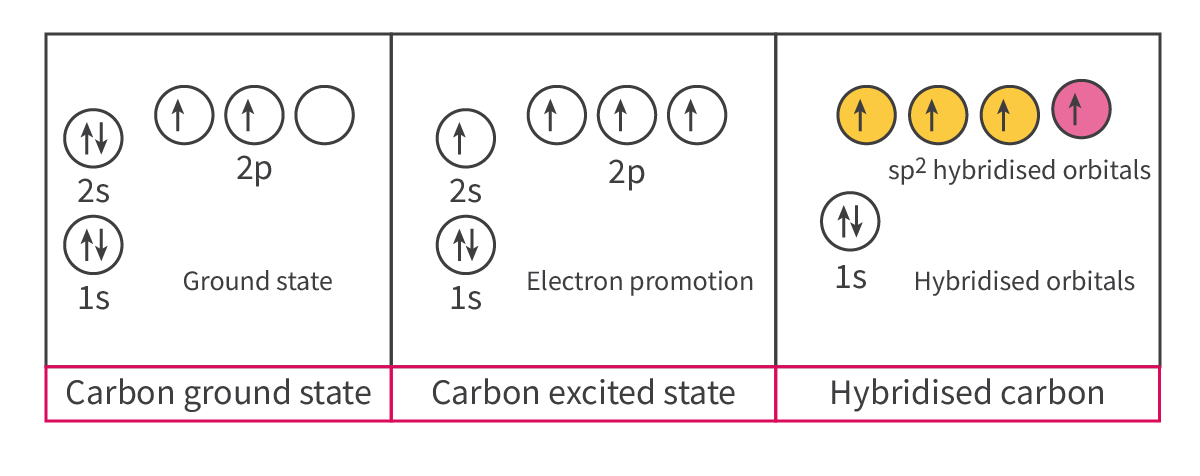
Sp2 hybridization:
- During double bonds:
○ There is 1 pi bond and 1 sigma bond (2 different bonds formed by different orbital overlaps, which DO NOT require the same level of hybridization)
○ Undergoes sp2 hybridization, where 1 s-orbital and 2 p-orbitals would become hybridized to produce 3 equal orbitals, but 1 of the 2p-orbitals is left unhybridized
§ This unhybridized 2p-orbital forms the pi bond, which is part of the double bond
The other 3 sp2 hybridized orbitals form sigma bonds
Sp2 hybridization:
- During ______ bonds:
○ There is ___ pi bond and ___ sigma bond (2 different bonds formed by different orbital overlaps, which DO NOT require the same level of hybridization)
○ Undergoes sp2 hybridization, where ___ s-orbital and ___ p-orbitals would become hybridized to produce 3 equal orbitals, but 1 of the 2p-orbitals is left _______
§ This unhybridized 2p-orbital forms the pi bond, which is part of the double bond
The other 3 sp2 hybridized orbitals form sigma bonds
Sp hybridization:
- During triple bonds:
○ Will undergo less hybridization
○ Only hybridize 2 of its orbitals, 1 of the s-orbital and 1 p-orbital
○ 2 hybridized sp-orbitals can form sigma bonds, and the unhybridized p-orbitals can form pi bonds
Sp hybridization:
- During _____ bonds:
○ Will undergo _____ hybridization
○ Only hybridize ____ of its orbitals, 1 of the s-orbital and 1 p-orbital
○ 2 hybridized sp-orbitals can form sigma bonds, and the unhybridized p-orbitals can form pi bonds
Linear, sp
State the arrangement and hybridization of: 2 regions of electron density
Trigonal planar, sp2
State the arrangement and hybridization of: 3 regions of electron density
Tetrahedral, sp3
State the arrangement and hybridization of: 4 regions of electron density
Covalent bond
Electrostatic attraction between a shared pair of electrons and the positively charged nuclei
Octet Rule
Tendency of atoms to gain a valence shell with a total of 8 electrons
Lewis Formula
Shows all valence electrons, whether these are pairs of electrons involved in covalent bonds or non-bonded pairs of electrons (non-bonding pairs)
Bond Strength
Measure of the energy required to break a bond
- More bonds = stronger the bond
Multiple bonds = Involve greater number of shared electrons = electrostatic attraction between them and positive nuclei is stronger = stronger bond

Bond Length
Measure of the distance between 2 bonded nuclei
Bond strength
C-N < C=N < C≡N
Bond length
C-N > C=N > C≡N
Coordination bonds
A covalent bond where both of the electrons being shared in the bond have been donated by one atom

To form a coordination bond:
One of the species in the bond must have non-bonding electrons available for bonding --> This atom is called "donor"
Coordination bonds allow atoms in a molecule to achieve stable configuration with 8 electrons in outermost shell = Accordance with octet rule
To form a coordination bond:
One of the species in the bond must have ___-_____ electrons available for bonding --> This atom is called "____"
Coordination bonds allow atoms in a molecule to achieve ____ configuration with 8 electrons in ______ shell = Accordance with octet rule
Donor to Receiver
Arrow in a coordination bond points from:
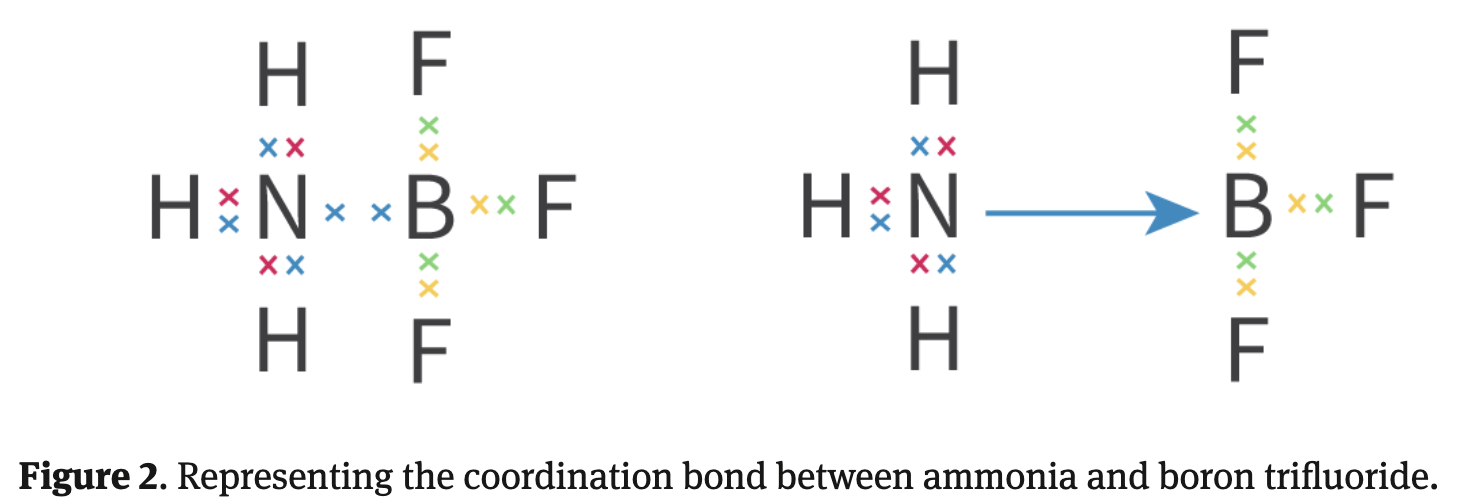
Central atom
A in the AXE
Bonding pairs of electrons
X in AXE
Non-bonding electron pairs
E in AXE
180° linear
AX
180° linear
AX2

180° linear
AXE
120° trigonal planar
AX3

<120° angular/bent
AX2E

180° linear
AXE2

109.5° tetrahedral
AX4

<109.5° triangular pyramidal
AX3E

«109.5 angular/bent
AX2E2

180° linear
AXE3

trigonal bipyramidal
AX5

Seesaw
AX4E

T-shaped
AX3E2

Linear 180°
AX2E3

Octahedral
AX6

Square pyramidal
AX5E
