Organic Chemistry
1/551
Earn XP
Description and Tags
Creating this from are dnga chapter then creating flash cards from what I think is important to know for the exam
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
552 Terms
1. identify parent chain
2. number the chain
3. name substituents
4. assign number to each substituent
5. complete name
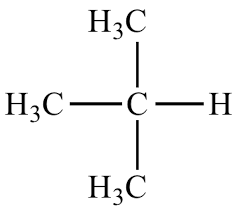
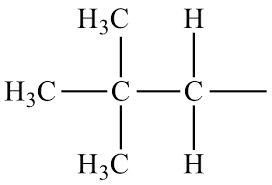
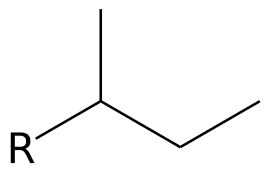
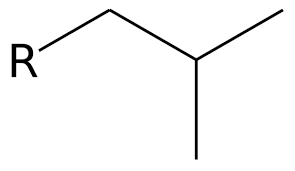
* number goes in front of substituent
* numbers are separated by commas
* words are separated by dashes
* finish with parent chain name including highest functional group
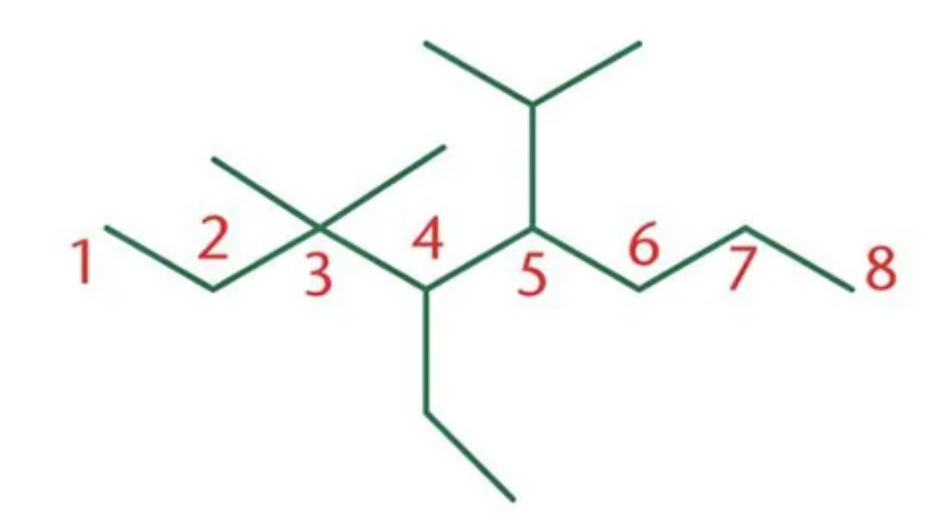
1. methane
2. ethane
3. propane
4. butane
5. pentane
6. hexane
7. heptane
1. octane
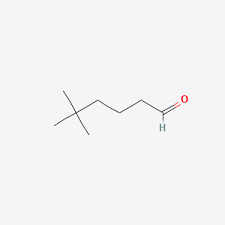
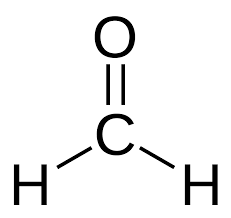
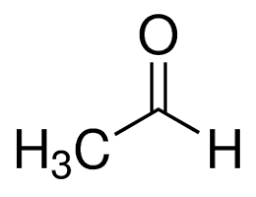
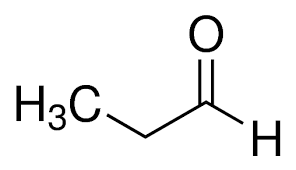
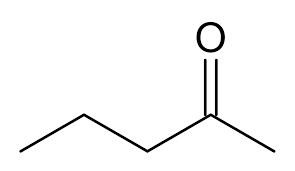
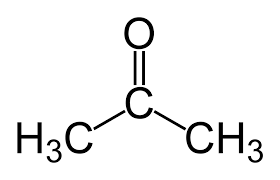
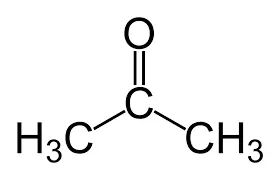
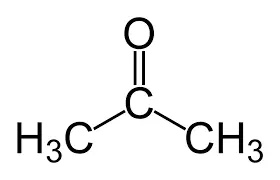
alpha ⍺ → beta β → gamma 𝛄 → delta 𝛅
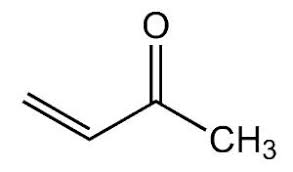
Common: methylvinylketone
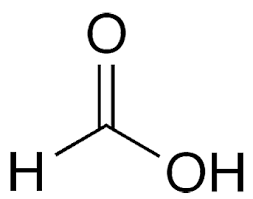
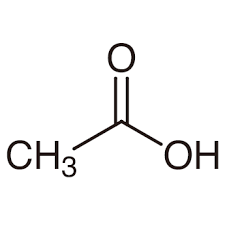
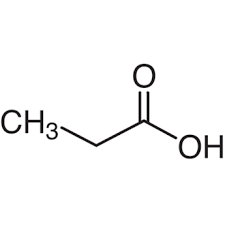
second term: parent acid with -oate instead of -oic acid
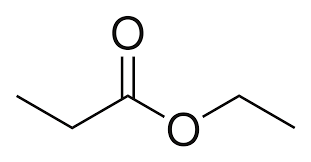
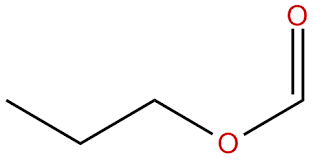
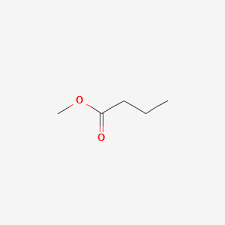
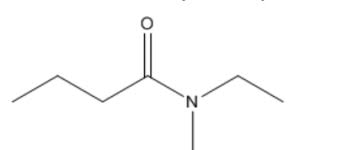
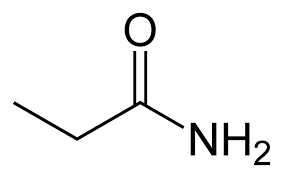
second term: parent acid with -amide suffix
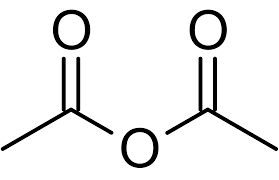
Common: acetic anhydride
1. carboxylic acid
2. anhydride
3. ester
4. amide
5. aldehyde
6. ketone
7. alcohol
8. alkene
9. alkyne
10. alkane
suffix: -oic acid
suffix: -anhydride
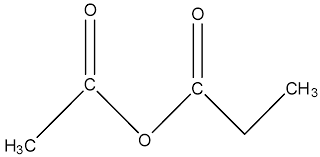
suffix: -oate
suffix: -amide
suffix: -al
suffix: -one
suffix: -ol
Suffix: -ene
suffix: -yl
suffix: -ane
What is a structural isomer?
Two molecules that have the same formula
What must be the same for structural isomers?
Molecular weight
For structural isomers, are chemical properties same or different?
different
For structural isomers, are physical properties same or different?
Different
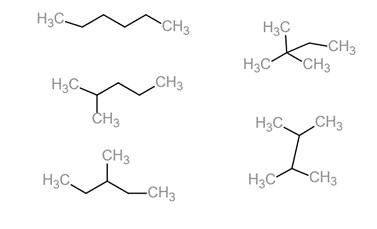
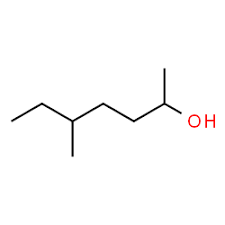
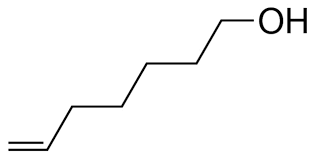
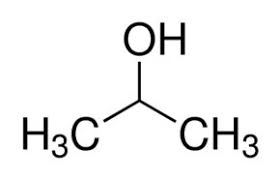
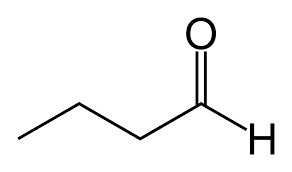
What kind of isomer are these?
Structural
What are stereoisomers?
share same atomic connectivity but differ in 3D orientation
What are conformational isomers?
molecules that differ in rotation around a single bond
What are configurational isomers?
molecules that can only be interconverted by breaking a bond
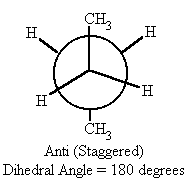
What is a good way to visualize conformational isomers?
Newman Projections
Where is a Newman projection visualized?
along line extending through C-C bond axis
What conformation is the lowest energy-state for straight-chain conformational isomers?
anti conformation
What is staggered conformation?
No overlap of atoms along line of sight
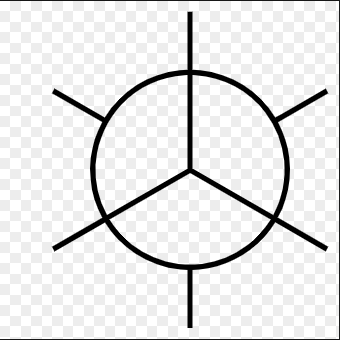
What is Anti Conformation?
Two largest groups are in same plane but on opposite sides