Alkene Addition Reactions
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
HBr
Hydrohalogenation
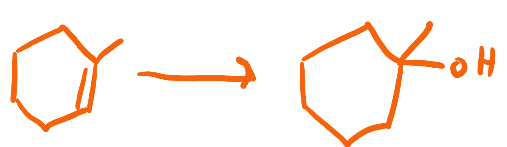
H2SO4, H2O
Acid Catalyzed Hydration (or Hydration)
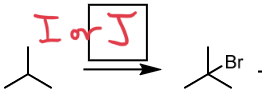
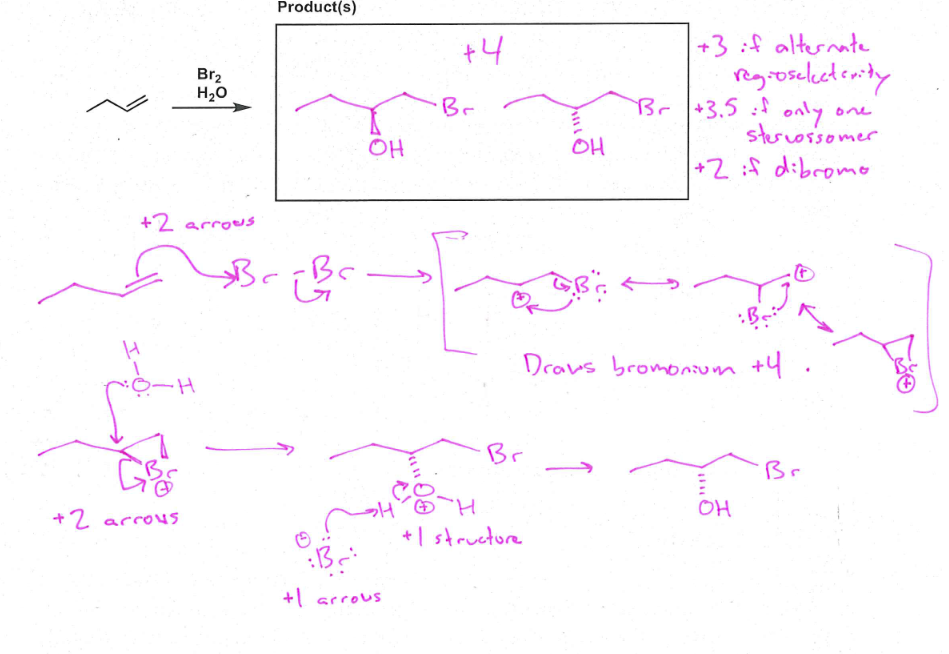
Cl2 or Br2
Halogenation
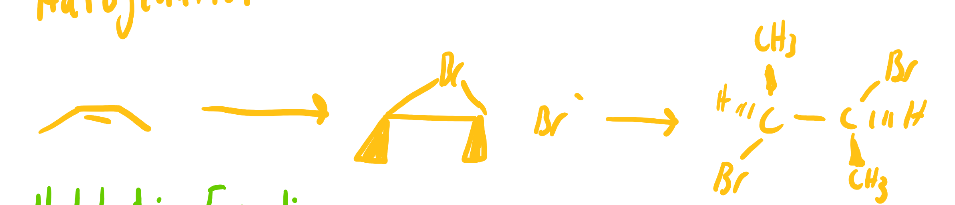
X2, H20
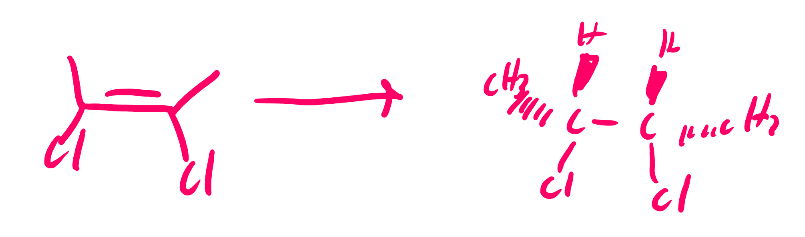
Halohydrin Formation
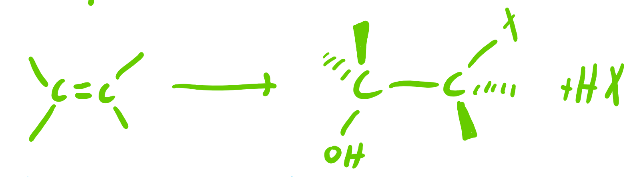
1) Hg(OAc)2, H2O
2) NaBH4
Oxymercuration-Reduction
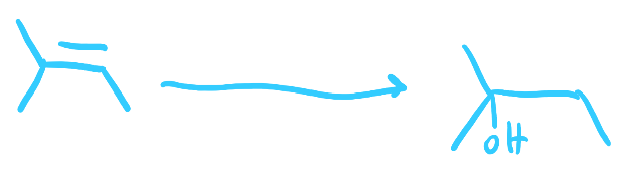
1. BH3, THF 2. H2O2, NaOH
Hydroboration-Oxidation
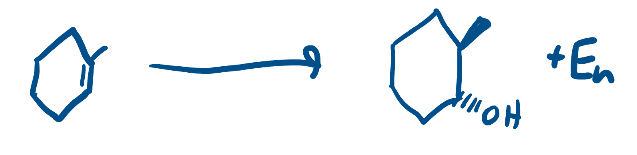
OsO4
Dihydroxylation
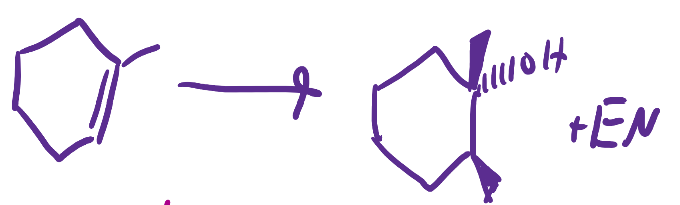
(1) O3; (2) S(CH3)2
Ozonolysis

addition of hydrogen and metal catalyst
(H2, Pd)
Hydrogenation
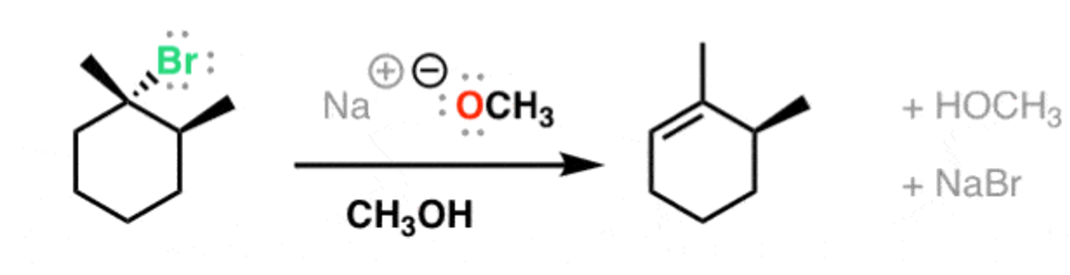
NaOCH3
E2
H2O
SN1
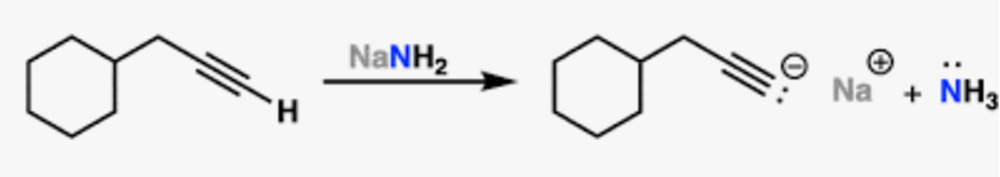
NaNH2
alkyne deprotonation
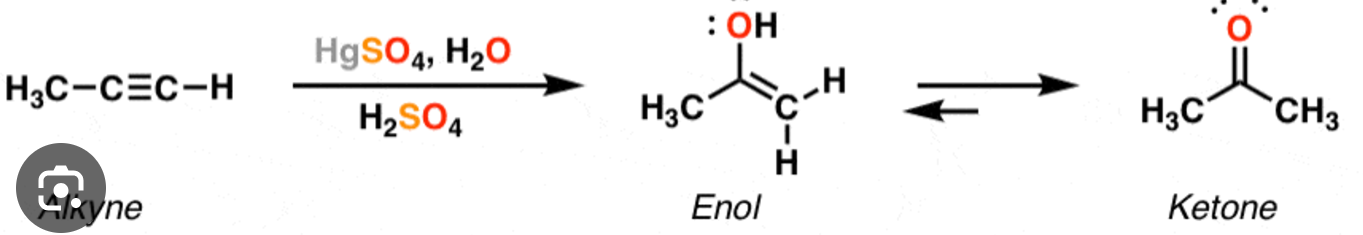
H2O, HgSO4, H2SO4
oxymercuration of alkynes
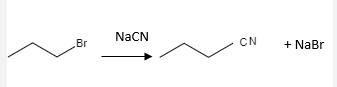
NaCN
SN2
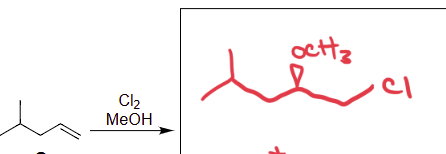
Cl2 MeOH
Halohydrin Formation
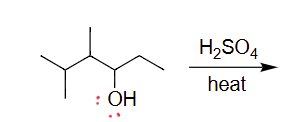
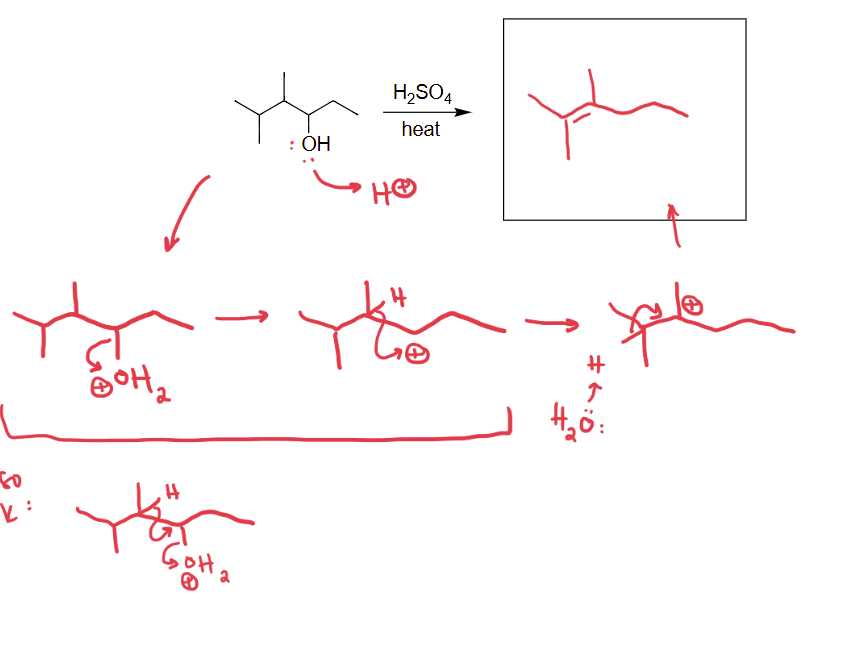
draw acid-catalyzes dehydration reaction
check
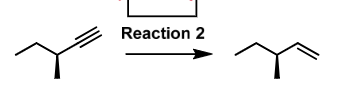
H2, Lindlar (cat.)
alkyne to alkene
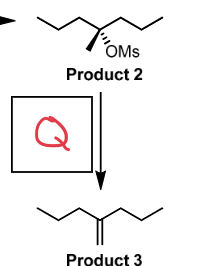
t-BuOK
E2, side group to alkene
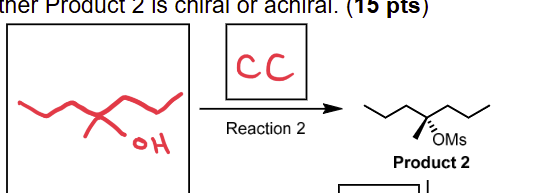
TsCl (or MsCl), pyridine (or Et 3N)
conversion of OH to OTs or OMs
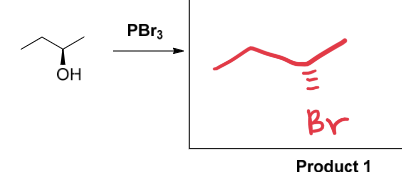
PBr3
SN2
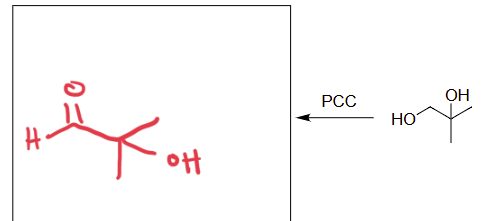
PCC
oxidation of alcohols
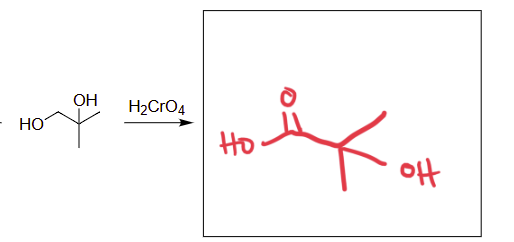
H2CrO4
oxidation of alcohols (creates carboxylic acid)
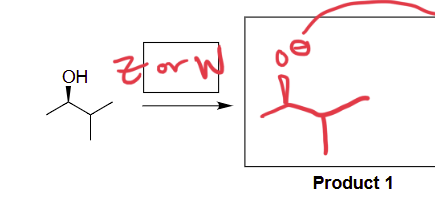
NaNH2 (on side group, no alkyne)
removes hydrogen
X 2 (Br2 or Cl2), heat or light
halogenation of alkanes
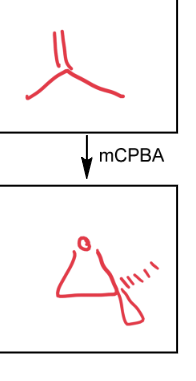
mCPBA
epoxidation of alkene
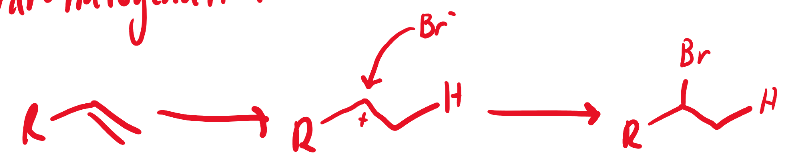
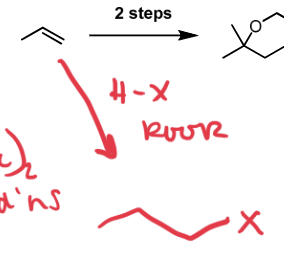
HBr, Peroxides
hydrohalogenation; anti-markovnikov
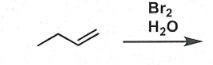
draw halohydrin formation reaction
check
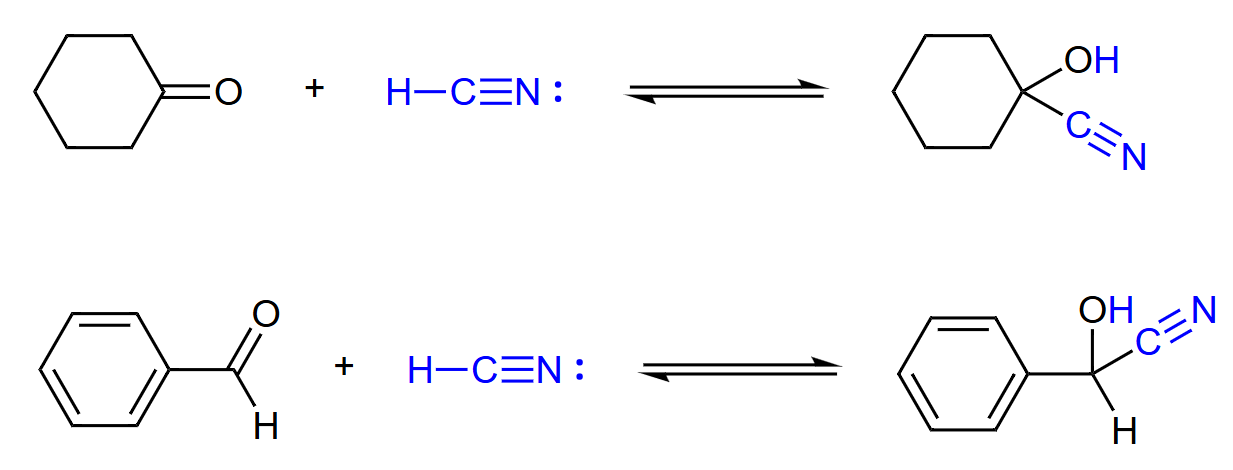
HCN
Cyanohydrin Formation
NBS, light
allylic bromination

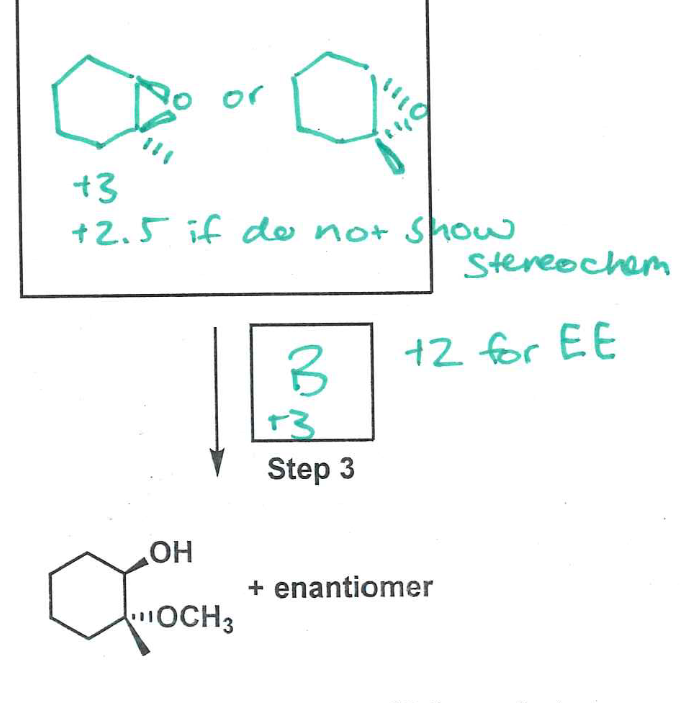
CH3OH, H2SO4
epoxi to OCH3
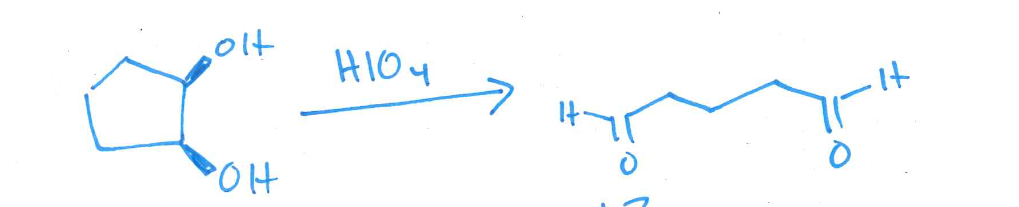
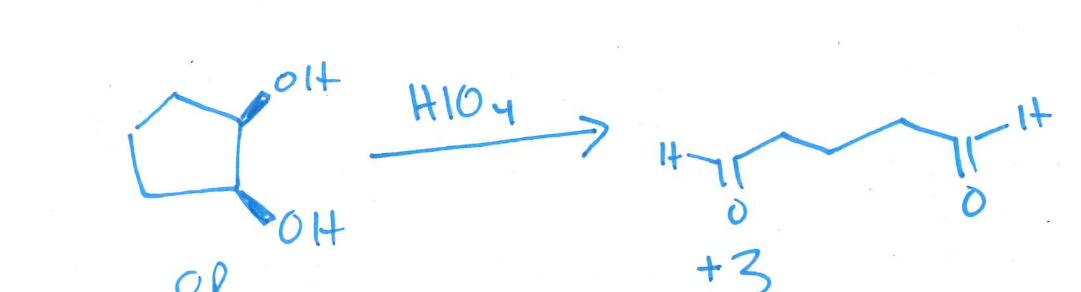
give the structure of the product of oxidative cleavage of cis-1,2-cyclopentanediol with HIO4. Draw both the starting material and the product
check
NaOH
strong base, E2
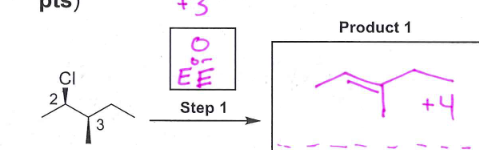
give the structure of the product of oxidative cleavage of cis-1,2-cyclopentanediol with HIO3. Draw both the starting material and the product
check