General Chemistry 4-6
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
90 Terms
What is the pH of a 0.001 M solution of sulfuric acid? (pKa = -2.8), assuming complete dissociation?
A. 0
B. 1
C. 2
D. 3
E. 4
3
Which of the following is the correct molecular geometry of PCl5?
A. Bent
B. Trigonal planar
C. Tetrahedral
D. Trigonal bipyramidal
E. Octahedral
Trigonal bipyramidal
Which of the following elements undergoes oxidation in the unbalanced equation for the chemical reaction below?
NaCl + H2SO4 + MnO2 → Na2SO4 + MnSO4 + H2O + Cl2
A. Oxygen
B. Manganese
C. Sulfur
D. Chlorine
E. Sodium
Chlorine
Which of the following bonds is the most polar?
A. C-H
B. O-F
C. C-C
D. C-N
E. O-H
O-H
Radioactive decay is the spontaneous breakdown of an atomic nucleus resulting in the release of energy and matter from the nucleus. What order kinetics does radioactive decay follow?
A. Zero-order kinetics
B. First-order kinetics
C. Second-order kinetics
D. Third-order kinetics
First-order kinetics
The distance separating two adjacent carbon nuclei is 1.54 Å and the bond length in F2 is 1.32 Å. Based on this data, which of the following is the length of the C-F bond in CF4?
A. 1.21
B. 1.36
C. 1.43
D. 1.54
E. 1.62
1.43
According to the following reaction, how many grams of Fe2O3 are formed when 15 g of iron is combusted?
4 Fe + 3 O2 → 2 Fe2O3
A.
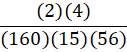
B.
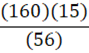
C.
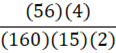
D.
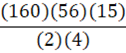
E.
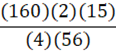
d
What is the heat of formation for one mole of ethane gas (C2H6) if ΔHfo for ethene gas (C2H4) is +50.5 kJ•mol-1?
C2H4(g) + H2(g) → C2H6(g) ΔH= -125.1 kJ
A. 26.4 kJ
B. -24.1 kJ
C. -74.6 kJ
D. -119.7 kJ
E. -175.6 kJ
-74.6 kJ
It takes 180 g of benzene at a density of 0.832 g•mL-1 to fill a container. At constant temperature, what mass of petroleum ether (0.64 g•mL-1) is needed to fill the same container?
A.
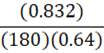
B.
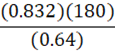
C.
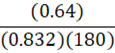
D.
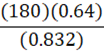
E.
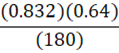
d
For the reaction below, which of the following sets of coefficients would balance the reaction?
C7H10N + O2 → CO2 + H2O + __NO2
A. 2, 24, 14, 10, 2
B. 2, 21, 14, 10, 2
C. 2, 18, 10, 10, 2
D. 2, 24, 10, 8, 2
E. 2, 21, 8, 12, 2
2, 21, 14, 10, 2
Elements that have 7 electrons in their outermost shell and commonly form salts during chemical reactions are collectively known as
A. metals.
B. non-metals.
C. inert gases.
D. noble gases.
E. halogens.
halogens.
Which of the following elements has the lowest electron affinity?
A. Li
B. B
C. O
D. F
E. Ne
Ne
Given 15 moles of nitrogen gas are present in an 8 L container at 25oC, what is the pressure of the nitrogen gas? (R = 8.314 L•kPa•K-1•mol-1)
A.
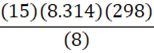
B.
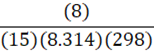
C.
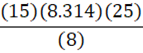
D.
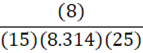
E.
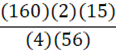
A
Which of the following is true for a reaction that has a small negative ΔG value?
A. Products will be favored at equilibrium
B. Reactants will be favored at equilibrium
C. No reactants will remain at equilibrium
D. No products will remain at equilibrium
E. No reaction would occur
Products will be favored at equilibrium
Which of the following best represent the decay process shown below?
238 | U | → | 234 | Th |
|
|
|---|---|---|---|---|---|---|
92 | 90 |
A. Alpha decay
B. β- decay
C. β+ decay
D. Electron capture
E. Gamma decay
Alpha decay
Which of the following would cause a random error?
A. pH meter calibrated incorrectly
B. Reading the meniscus at a different angle each time
C. Spilling the product
D. Electronic balance tarred incorrectly
E. Thermometer reading 103°C in pure boiling water
Reading the meniscus at a different angle each time
A 35 g metal alloy at 25oC absorbs 2.5 kJ of energy, raising its temperature to 35oC. What is the specific heat of the alloy, in J•g•-1•oC-1?
A.
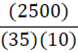
B.
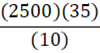
C.
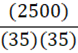
D.
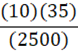
E.
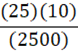
a
At which point during a titration experiment does pH = pKa ?
A. Half-equivalence point
B. Equivalence point
C. Triple point
D. Second equivalence point
E. Critical point
Half-equivalence point
What is the ionic reaction for the neutralization of HCl with NaOH?
A. HCl(aq) + NaOH(aq) ↔ H2O(l) + NaCl(aq)
B. H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) ↔ H+(aq) + OH-(aq) + Na+(aq) + Cl-(aq)
C. H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) ↔ H2O(l) + Na+(aq) + Cl-(aq)
D. H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) ↔ H2O(l) + NaCl(s)
E. H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) ↔ H+(aq) + O2(g) + Na+(aq) + Cl-(aq)
c
What process occurs when the vapor pressure of a liquid equals the atmospheric pressure?
A. Melting
B. Freezing
C. Boiling
D. Sublimation
E. Condensation
Boiling
Each of the following pairs of compounds forms hydrogen bonds with each other EXCEPT one. Which one is the EXCEPTION?
A. CH3CH2OH and H2O
B. NH2CH3 and CO
C. NH and HF
D. H2O and H2O2
E. H2S and HCl
H2S and HCl
If the temperature of a gas is halved at constant pressure, the volume
A. will be halved.
B. will be reduced to a third.
C. will triple.
D. will double.
E. will not change.
will be halved.
At 37°C, which one of the following solutions produces the lowest osmotic pressure?
A. 0.90 M fructose
B. 0.40 M MgSO4
C. 0.40 M (NH4)2SO4
D. 0.32 M Na2SO4
E. 1.0 M sucrose
0.40 M MgSO4
Based on the data shown below, what is the initial activity of the radioactive sample at the start (zero hours) if the half-life is 12 hours?
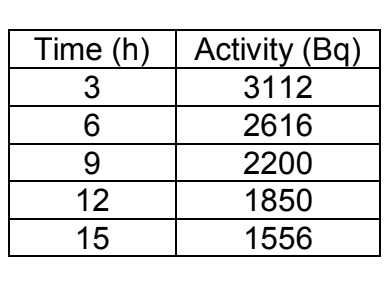
A. 3,437
B. 3,525
C. 3,650
D. 3,700
E. 3,835
3700
Which of the following gas laws states that two gases with equal volumes will have an equal number of molecules at the same temperature and pressure?
A. Boyle’s Law
B. Dalton’s Law
C. Gay-Lussac’s Law
D. Charles’ Law
E. Avogadro’s Law
Avogadro’s Law
The reaction of oxalate ions (C2O42-) with permanganate ions (MnO4-) in acidic solution forms carbon dioxide (CO2) and manganese(II) ions (Mn2+). What is the stoichiometric coefficient in front of oxalate ion in the balanced redox equation?
A. 2
B. 3
C. 5
D. 7
E. 10
5
The atomic theory states that matter is composed of
A. particles.
B. elements.
C. molecules.
D. atoms.
E. compounds.
atoms
How many equivalent resonance structures can be drawn for the nitrate ion, NO3-?
A. 1
B. 2
C. 3
D. 4
E. 5
3
Each of the following reactions has an equilibrium point that is affected by changes in pressure EXCEPT one. Which one is the EXCEPTION?
A. Li2S(aq) + HBr(aq) → 2 LiBr(aq) + H2S(g)
B. 2 H2(g) + O2(g) → 2 H2O(g)
C. 2 Na(s) + Cl2(g) → 2 NaCl(s)
D. C(s) + H2O(g) → CO(g) + H2(g)
E. CS2(l) + 3 O2(g) → CO2(g) +2 SO2(g)
CS2(l) + 3 O2(g) → CO2(g) +2 SO2(g)
In which of the following compounds does sulfur have an oxidation number of +2?
A. S2O32-
B. SO2
C. S2O62-
D. SO42-
E. H2S
S2O32-
Which of the following is true regarding pi bonds and sigma bonds?
A. Pi bonds are stronger than sigma bonds
B. Sigma bonds are formed by overlapping lateral orbitals
C. Pi bonds allow for rotation
D. A triple bond contains one sigma and two pi bonds
E. Pi bonds are formed by end-to-end overlapping orbitals
A triple bond contains one sigma and two pi bonds
Aqueous NH3 was titrated with HBr until the endpoint. The resulting solution underwent testing using four pH indicators, and the table below provides information about the indicators and the colors observed during the test. Based on the information provided, which of the following statements is correct?
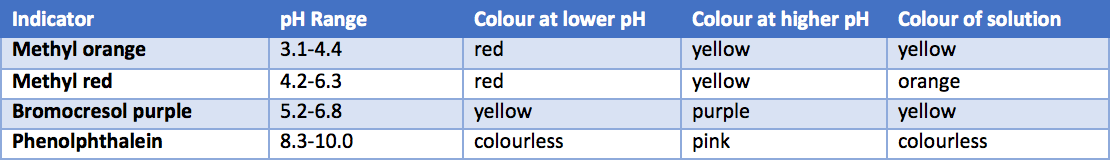
A. pH at endpoint is 4.8
B. pH at endpoint is 6.1
C. pH at endpoint is 12.0
D. pH at endpoint is 2.4
E. pH at endpoint is 7.0
pH at endpoint is 4.8
Which of the following best describe bond energy?
A. Energy required for a reaction to proceed
B. Energy required to break a bond
C. Energy required to create a bond
D. Energy required to maintain a bond
Energy required to break a bond
If it takes 95 g of diesel at a density of 0.832 g•mL-1 to fill a container, at constant temperature, what mass of benzene (0.740 g•mL-1) is needed to fill the same container?
A.
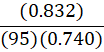
B.
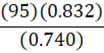
C.
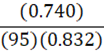
D.
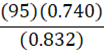
E.
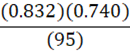
d
How many protons, neutrons, and electrons does the following anion have?
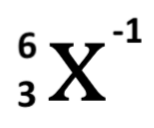
A. 4 protons, 3 neutrons and 4 electrons
B. 6 protons, 6 neutrons and 7 electrons
C. 6 protons, 3 neutrons and 4 electrons
D. 3 protons, 3 neutrons and 4 electrons
E. 3 protons, 4 neutrons and 4 electrons
3 protons, 3 neutrons and 4 electrons
All of the following are true regarding Galvanic cells EXCEPT one. Which one is the EXCEPTION?
A. Oxidation occurs at the anode
B. Galvanic cells produce chemical energy
C. Electrons flow from the anode to cathode
D. The salt bridge keeps solutions electrically neutral
E. Metal electrodes gain electrons at the cathode
Galvanic cells produce chemical energy
According to the following reaction, how many grams of Fe2O3 are formed when 383 g of iron is combusted?
4 Fe + 3 O2 → 2 Fe2O3
A.
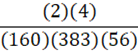
B.
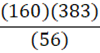
C.
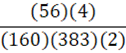
D.
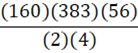
E.
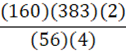
e
Given 5 moles of oxygen gas are present in a 5 L container at 25oC, what is the pressure of the oxygen gas? (R = 8.314 L•kPa•K-1•mol-1)
A. 2,300 kPa
B. 2,400 kPa
C. 2,500 kPa
D. 2,600 kPa
E. 2,700 kPa
2,500 kPa
Each of the following are ionic compounds EXCEPT one. Which one is the EXCEPTION?
A. NH4NO2
B. KH
C. LiNO3
D. CH3NH2
E. NaOH
CH3NH2
Which of the statements is true for a reaction with a negative ΔH?
A. The reaction will be endothermic
B. The reaction will be non-spontaneous
C. The reaction will be spontaneous
D. The reaction will be exothermic
E. The reaction will not occur
The reaction will be exothermic
How many electrons are involved in the following acidic redox reaction?
MnO4- + H2SO3 + H+ → Mn2+ + HSO4- + H2O
A. 2
B. 4
C. 6
D. 8
E. 10
10
How much energy is needed to bring 250 mL of water (4.18 J•g-1•oC-1) at 25oC to a boil?
A.
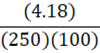
B.
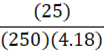
C.
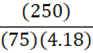
D.
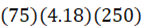
E.
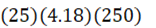
d
Which of the following is the correct electron configuration for the bromide ion, Br- ?
A. [Ar] 4s24d5
B. [Ar] 4s24d104p6
C. [Ar] 4s23d104p6
D. [Ar] 4s23d104p65s1
E. [Ar] 4s23d103p6
[Ar] 4s23d104p6
What is the empirical formula of a compound composed of 50.05% sulfur and 49.95% oxygen?
A. SO2
B. SO3
C. SO4
D. S2O4
E. S2O5
SO2
Given methanol has a density of 0.792 g•cm-3 at 273 K, what volume of container is needed to hold 0.060 kg of methanol at 273 K?
A. 65 cm3
B. 70 cm3
C. 75 cm3
D. 80 cm3
E. 85 cm3
75 cm3
Which of the following represents the highest value of pressure?
A. 100 kPa
B. 1 atm
C. 780 mmHg
D. 25 torr
E. 96,000 Pa
780 mmHg
Nitrogen gas, hydrogen gas, and ammonia gas were put into a chamber and allowed to react until equilibrium was reached. The reaction is outlined by the following equation:
N2(g) + 3 H2(g) → 2 NH3(g)
If the pressure at equilibrium was more than the initial pressure, what can be said about the reaction quotient and the direction of reaction as the reaction progressed toward equilibrium?
A. Qc > Kc ; forward reaction
B. Qc > Kc ; backward reaction
C. Qc < Kc ; forward reaction
D. Qc < Kc ; backward reaction
E. Qc = Kc ; backward reaction
Qc > Kc ; backward reaction
Which of the following compounds is miscible in water?
A. C6H6
B. CH3COCH3
C. CH3CH2CH3
D. CCl4
E. CH4
CH3COCH3
Based on the data shown below, what is the rate law for the following chemical reaction?
A + B → C
Experiment | [A] | [B] | Initial rate (M • s-1) |
|---|---|---|---|
1 | 0.1 M | 0.1 M | 2.0 × 10-5 |
2 | 0.2 M | 0.1 M | 8.0 × 10-5 |
3 | 0.1 M | 0.2 M | 4.0 × 10-5 |
A. Rate = k[A][B]
B. Rate = k[A][B]2
C. Rate = k[A]2[B]
D. Rate = k[A]2[B]2
E. Rate = k[B]
Rate = k[A]2[B]
Given the titration curve below, what quantity of 0.1 M HCl is required to reach the equivalence point in the titration with 0.1 M of NH3?
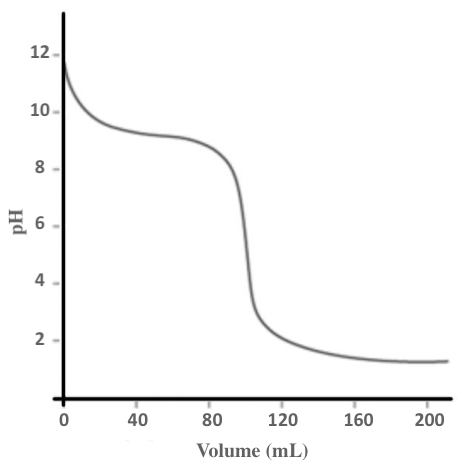
A. 9 mmol
B. 10 mmol
C. 12 mmol
D. 16 mmol
E. 18 mmol
10 mmol
How many moles of oxygen are in a 190 g solution of KNO3 at a temperature of 28oC ?
A. 4.8
B. 5.1
C. 5.4
D. 5.7
E. 6.0
5.7
Which of the following compounds contains nitrogen with an oxidation number of -2?
A. N2H5+
B. NH3
C. N2O
D. NO
E. HNO2
N2H5+
In a fission reaction, uranium-235 is bombarded with a neutron and decays into two main radioactive fragments and 3 neutrons as depicted in the following reaction:
235U + 1n → 141Ba + X + 31n
What is the element and the atomic mass of radioactive fragment X?
A. 92U
B. 92Kr
C. 95Kr
D. 92Ba
92Kr
Each of the following electron configurations obeys Hund’s Rule EXCEPT one. Which one is the EXCEPTION?
A.
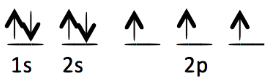
B.
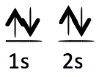
C.
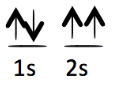
D.
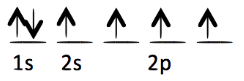
E.
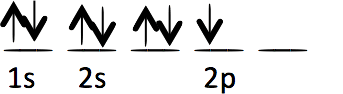
E
Using an incorrectly calibrated pipette will result in
A. gross error.
B. random error.
C. systematic error.
D. personal error.
E. method error.
systematic error.
Which of the following represents the solubility product constant for lead chloride?
PbCl2(s) ⇌ Pb2+(aq) + 2 Cl-(aq)
A. [Pb2+][Cl-]
B. [Pb2+][Cl-]2
C. 2[Pb2+][Cl-]
D. 2[Pb2+][Cl-]2
E. [Pb2+]2[Cl-]
[Pb2+][Cl-]2
Given the enthalpy data for reactions shown below, what is the enthalpy change for the following reaction?
N2(g) + 2 O2(g) → 2 NO2(g)
2 NH3(g) + 4 H2O(l) → 2 NO2(g) + 7 H2(g) ΔH = -140 kJ
2 NH3(g) → N2(g) + 3 H2(g) ΔH = 110 kJ
H2O(l) → H2(g) + ½ O2(g) ΔH = -50 kJ
A. -100 kJ
B. -50 kJ
C. 50 kJ
D. 100 kJ
E. 250 kJ
-50 kJ
For the reaction below, which of the following sets of coefficients would balance the reaction?
Ca3(PO4)2 + SiO2 + C → CaSiO3 + P4 + CO2
A. 1, 3, 6, 6, 1, 6
B. 1, 3, 5, 6, 1, 5
C. 1, 3, 5, 3, 1, 5
D. 2, 6, 5, 6, 1, 5
E. 2, 6, 10, 6, 1, 10
2, 6, 5, 6, 1, 5
What does the azimuthal quantum number represent?
A. Orbital size and energy level
B. Quantity of electrons in an orbital
C. Electron spin direction
D. Shape of the orbital
E. Orbital orientation
Shape of the orbital
A Lewis acid is
A. a proton producer.
B. a proton donor.
C. a proton acceptor.
D. an electron acceptor.
E. an electron donor.
an electron acceptor.
A container with a tightly fitted movable piston contains carbon dioxide gas at 27oC. What is the change in internal energy of the gas if 200 J of heat enters the gas and the gas does 300 J of work in the process?
A. -500 J
B. -100 J
C. 0 J
D. +100 J
E. +500 J
-100 J
What is the change in boiling point when 5 M of NaCl is dissolved in water? (Kb water = 0.51 oC•kg•mol-1)
A. –(2)(0.51)(5)
B. (0.51)(5)
C. (2)(0.51)(5)
D. –(0.51)(5)
E. (2)(0.51)(10)
(2)(0.51)(5)
Nobelium-259 is a radioactive isotope with a half-life of 60 minutes. If a nobelium-259 sample weighs 3.75 g after 3 hours, how many grams of Nobelium-259 were in the original sample?
A. 1.875 g
B. 7.5 g
C. 15 g
D. 30 g
E. 60 g
30 g
Which of the following represents the equilibrium constant for the weak base, NH3 in an aqueous solution?
A.
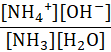
B.
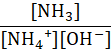
C.
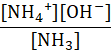
D.
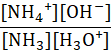
E.
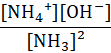
c
Given that the rate law for a certain reaction is rate = k[A][B], what is the unit of the rate constant?
A. s-1
B. M-1
C. M-1s-1
D. M-1s-2
E. M-2s-1
M-1s-1
What is the osmotic pressure of a 1.5 M sucrose solution at 300 K?
A. 3.7 atm
B. 5.5 atm
C. 37 atm
D. 71 atm
E. 74 atm
37 atm
Which of the following represents the Ksp of AgCl if the molar solubility is 1.3 × 10-5?
A. (1.3 × 10-5)(1.3 × 10-5)
B. (1.3 × 10-5)(1.3 × 10-5)2
C. (1.3 × 10-5)(2)(1.3 × 10-5)2
D. (1.3 × 10-5)2(1.3 × 10-5)2
E. (1.3 × 10-5)(2)(1.3 × 10-5)
(1.3 × 10-5)(1.3 × 10-5)
If a gas mixture containing a total of 10 moles has partial pressures of 0.5 atm for O2, 1.4 atm for N2, and 0.6 atm for F2, how many moles of oxygen gas are present in the mixture?
A. 2
B. 2.5
C. 3.5
D. 5
E. 8
2
Which of the following is true for a reaction with ΔH < 0 J and ΔS < 0 J•K-1?
A. The reaction is spontaneous at all temperatures
B. The reaction is non-spontaneous at all temperatures
C. The reaction is spontaneous at low temperatures
D. The reaction is spontaneous at high temperatures
The reaction is spontaneous at low temperatures
According to the following reaction, how many grams of hydrogen gas are formed when 54 g of aluminum reacts with 49 g of sulfuric acid?
2 Al + 3 H2SO4 → Al2(SO4)3 + 3 H2
A. 1 g
B. 2 g
C. 3 g
D. 4 g
E. 5 g
1 g
Weaker intermolecular forces result in a higher
A. melting point.
B. vapor pressure.
C. viscosity.
D. surface tension.
E. boiling point.
vapor pressure.
Acetic acid and its conjugate base acetate (Ka = 1.8 × 10-5) can be used to make a simple buffer solution. Which of the following pH indicators would be suitable for monitoring the pH while making this buffer?
A. Methyl yellow (pH 2.9 – 4.0)
B. Congo red (pH 3.0 – 5.0)
C. Bromocresol purple (pH 5.2 – 6.8)
D. Neutral red (pH 6.8 – 8.0)
E. Cresol red (pH 7.2 – 8.8)
Congo red (pH 3.0 – 5.0)
The phase diagram for an unknown compound is given below. If the compound is initially at point A before the pressure is decreased, what is the first phase change that the compound will undergo?
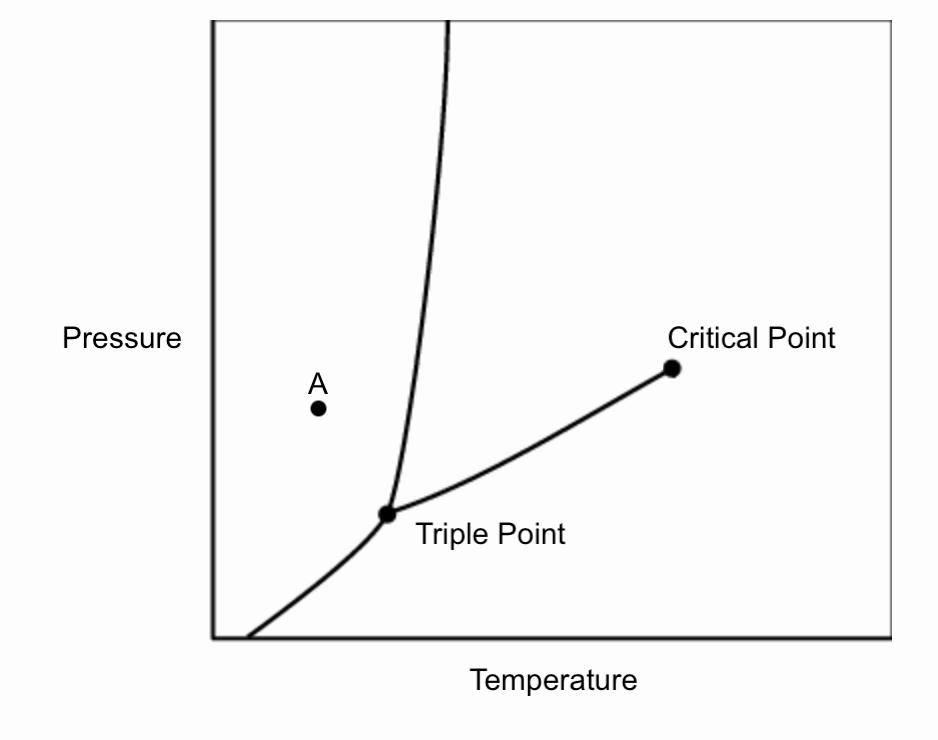
A. Deposition
B. Fusion
C. Crystallization
D. Vaporization
E. Sublimation
Sublimation
What is the osmotic pressure for 0.20 M of Na3PO4 at 30oC?
A. (0.2)(0.0821)(303)
B. (2)(0.2)(0.0821)(303)
C. (4)(0.2)(0.0821)(30)
D. (4)(0.2)(303)
E. (4)(0.2)(0.0821)(303)
(4)(0.2)(0.0821)(303)
Which of the following is TRUE for reaction coordinates?
A. The difference between the reactant and product energy is the activation energy
B. Energy released in exergonic reactions is the energy difference between products and reactants
C. An intermediate occurs at an energy maximum
D. The difference between the reactant energy and the transition state is the enthalpy
E. The relaxation energy is the negative of the activation energy
Energy released in exergonic reactions is the energy difference between products and reactants
Which of the following is a rate expression for the following reaction?
2 H2O(g) → 2 H2(g) + O2(g)
A. [H2O] / 2Δt
B. -Δ [H2] / 2Δt
C. -Δ [O2] / 2Δt
D. -Δ [H2O] / Δt
E. Δ [O2] / Δt
Δ [O2] / Δt
Each of the following is a Bronsted-Lowry conjugate acid-base pair EXCEPT one. Which one is the EXCEPTION?
A. HPO32- , PO33-
B. CH3CH2CO2H , CH3CH2CO2-
C. HNO3 , HNO2
D. NH3 , NH2–
E. H2S , HS-
HNO3 , HNO2
What is the heat of formation for 2 moles of N2O5 gas based on the equations below?
2 N2(g) + 5 O2(g) → 2 N2O5(g) ΔH = ?
H2(g) + ½ O2(g) → H2O(ℓ) ΔH = -250 kJ
N2O5(g) + H2O(ℓ) → 2 HNO3(ℓ) ΔH = -50 kJ
N2(g) + 3 O2(g) + H2(g) → 2 HNO3(ℓ) ΔH = -350 kJ
A. -100 kJ
B. -150 kJ
C. 50 kJ
D. 650 kJ
E. -50 kJ
-100 kJ
Which of the following is the percent composition of oxygen in glucose, C6H12O6?
A.
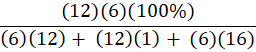
B.
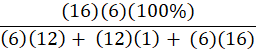
C.
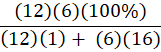
D.
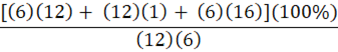
E.
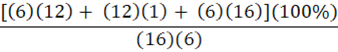
B
A student performs an experimental reaction and recovers 15 g of the product. Based on the amount of reactant used, it was estimated that 20 g of the product should have been obtained. What is the yield of the reaction?
A. (15)/(20)
B. (20-15)/(20)
C. (20-15)/(15)
D. (20)/(15)
E. (15-20)/(20)
(15)/(20)
Each of the following are strong acids EXCEPT one. Which one is the EXCEPTION?
A. HClO4
B. HBr
C. HF
D. H2SO4
E. HClO3
HF
Which of the following molecules is correctly matched with its bond angle?
A. XeF2 (120º)
B. SF6 (107.4º)
C. SO42- (<104º)
D. H2CO (120º)
E. H2O (109º)
A. XeF2
B. SF6
C. SO42-
D. H2CO
E. H2O
H2CO
If 0.92 g of toluene was used to lower the freezing point of 100 g of benzene by 0.50 oC (Kf benzene = 5oC•m-1), what is the estimated molar mass of toluene used?
A. 75 g•mol-1
B. 80 g•mol-1
C. 92 g•mol-1
D. 90 g•mol-1
E. 95 g•mol-1
92 g•mol-1
If the volume of an ideal gas is doubled and the temperature is tripled, what change does the pressure undergo?
A. 1/6 of the original pressure
B. 2/3 of the original pressure
C. 6 times the original pressure
D. 3/2 of the original pressure
E. 3 times the original pressure
3/2 of the original pressure
Which of the following elements would be strongly attracted to a magnetic field?
A. Ga
B. Zn
C. Ca
D. Mg
E. Ne
Ga
Which of the following molecules has the strongest dipole moment?
A. PF5
B. CCl4
C. PBr3O
D. CO2
E. CH4
PBr3O
Which of the following pairs correctly represents the heat of fusion and heat of vaporization, respectively, for a 36-gram sample of water that requires 102.6 kJ of energy to undergo sublimation?
A. 25.65 kJ•mol-1; 25.65 kJ•mol-1
B. 20.2 kJ•mol-1; 82.4 kJ•mol-1
C. 42.3 kJ•mol-1; 9.0 kJ•mol-1
D. 9.0 kJ•mol-1; 42.3 kJ•mol-1
E. 82.4 kJ•mol-1; 20.2 kJ•mol-1
9.0 kJ•mol-1; 42.3 kJ•mol-1
Which of the following molecules has a linear molecular geometry?
A. PCl3
B. CH3Cl
C. XeF2
D. H2O
E. O3
XeF2
Given a reaction with ΔH = 50 kJ and ΔS = 25 J•K-1, at what temperature will the reaction become spontaneous?
A. Above 2,000°C
B. Below 2,000 K
C. Above 2,000 K
D. Below 2,000°C
E. Above 2,273 K
Above 2,000 K
Which of the following statements best explains why sodium is more reactive than magnesium?
A. Sodium has a lower atomic radius
B. Sodium has a lower electronegativity
C. Sodium is a metal
D. Group 1 elements are not very reactive
E. Sodium has a higher ionization energy
Sodium has a lower electronegativity