IMED1003 - Electron Transport Chain and Oxidative Phosphorylation (L12)
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
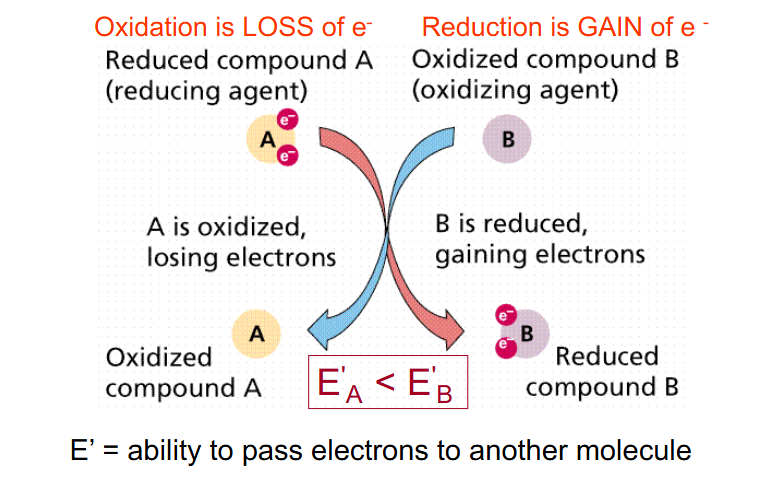
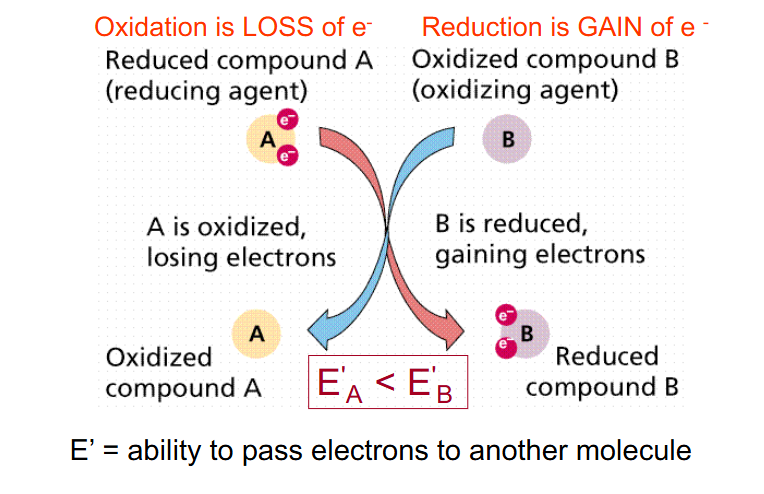
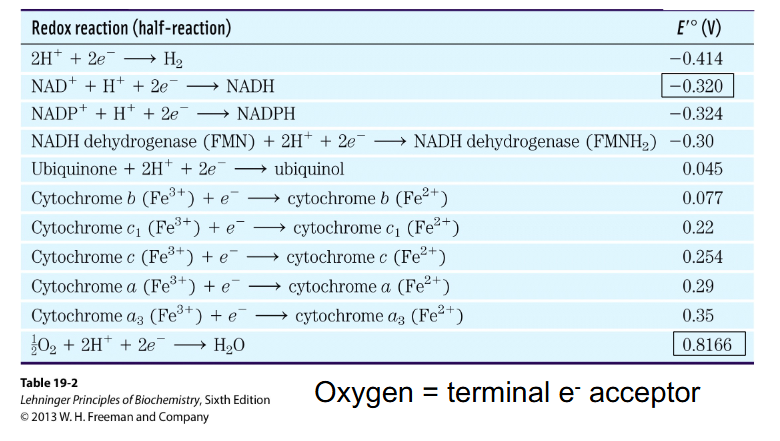
E' = Redox Potential
- measure of ability of one molecule to pass electrons to another
- more negative E' indicates stronger reductant - so more readily DONATES electrons
NADH E' = -0.32V
Oxygen E' = +0.82V
- NADH is at a higher energy level then oxygen (it wants to donate electrons more)
- when e- pass from a compound with more negative redox potential to one of more positive redox potential, energy is released
- oxygen is the terminal (last) electron acceptor of respiration (in the electron transport chain)
Redox Potential
- Oxidation is loss of e-
- Reduction is gain of e-
E' = ability to pass electrons to another molecule
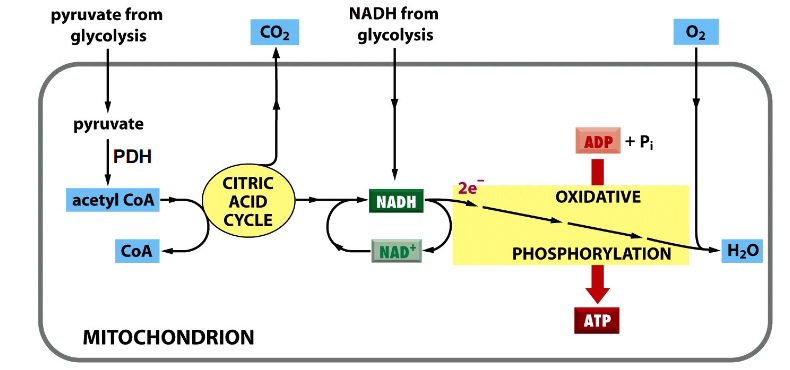
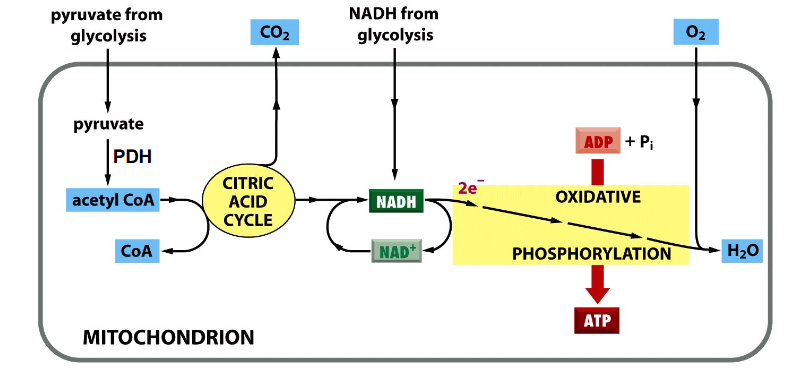
Process of Cellular Respiration
DIAGRAM ON SLIDE 5
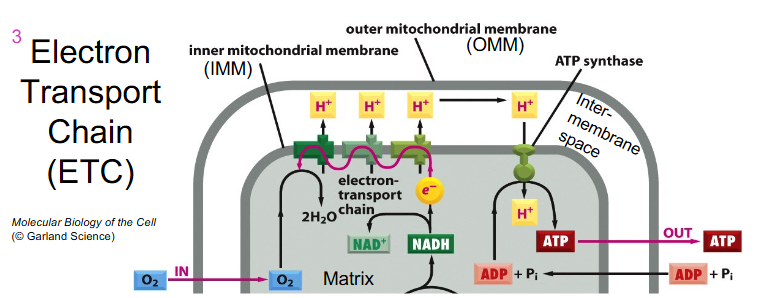
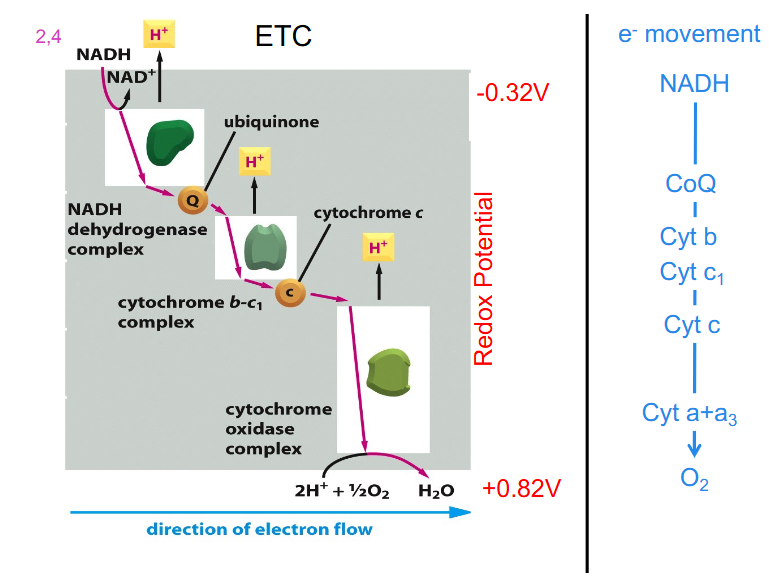
Electron Transport Chain (ETC)
- ETC = sequentially acting e- carriers, most are proteins with prosthetic groups able to accept and donate e- (e.g directly as e-, as H atom or hydride ion)
- Ubiquinone = CoQ is lipid-soluble and mobile e- carrier (it can physically move in the IMM)
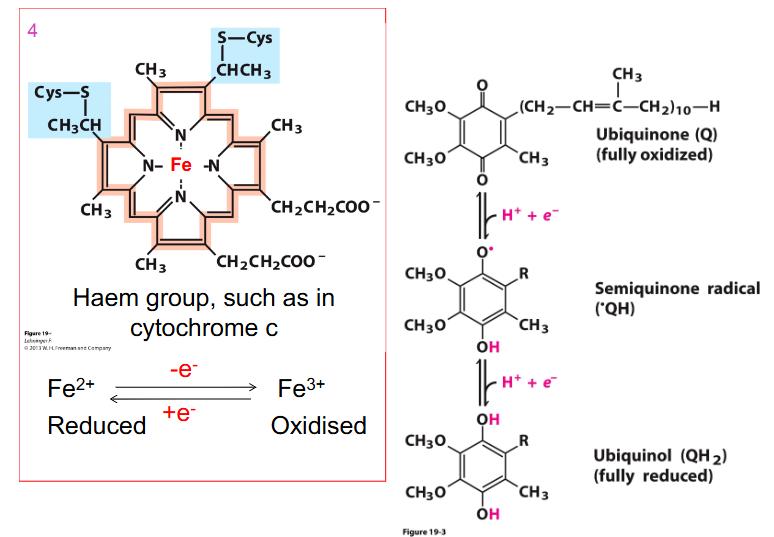
- Cytochromes (a, b, c) = iron-containing proteins (haem prosthetic group)
- can be reduced (Fe2+) or oxidised (Fe3+)
- Cytochrome C is mobile
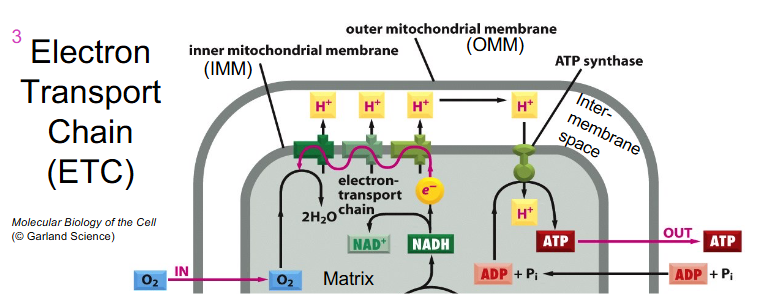
Electron Transport Chain Key Info
- Located in the inner mitochondrial membrane
- Series of specialised acceptor and donor molecules
- electron carriers, 3 of which act as proton pumps
PROTEIN COMPLEX:
1: NADH Dehydrogenase
2: Succinate dehydrogenase (one of two entry points for electrons, acquiring electrons from succinate and donating them to ubiquinone (CoQ))
3: Ubiquinone: cytochrome C oxidoreductase (Cytocrhome bc1)
4: Cytochrome oxidase
5: ATP Synthase
- prosthetic groups which allow e- movement. e.g Haem
- complexes 1, 3 and 4 pump protons out of matrix, across IMM (inner mitochondrial membrane) into intermembrane space
- they all have a component within them that can be reduced as they accept electrons and then oxidise as they donate the electron to the next carrier
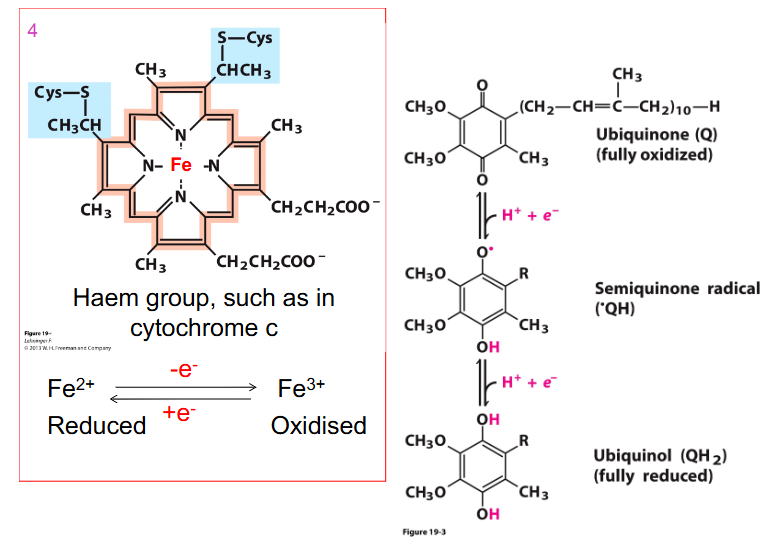
Cytochrome
- contains the metal iron
- one of the roles of iron is to be in these components of the electron transport chain, because iron can be Fe2+ or Fe3+ so it can be easily oxidised and reduced backwards and forwards by accepting and donating electrons
Point of Electron Transport Chain
- the point is to generate a chemical and electrical gradient that allows us to activate ATP synthase and therefore generate ATP
- as e- pass along the chain, they fall to successively lower energy levels (more +ve E')
- energy released pumps protons out of the matrix, across the inner mitochondrial membrane and into the intermembrane space - proton gradient is generated
- Source of energy (like a battery) which can be tapped to drive a variety of energy requiring reactions, e.g generation of ATP from Pi and ADP (oxidative phosphorylation) by ATP synthase
- At the end, O2 is reduced to produce water (H2O). Thus, O2 is the final electron acceptor of cellular respiration
- the electron transport chain can only work if there is oxygen, becuase oxygen
Why O2 is needed
If we don't have oxygen, we cannot oxidise NADH to NAD+, which means NADH will accumulate and it will start to shut down the TCA Cycle
- it will start to shut down pyruvate dehydrogenase
- cell will die
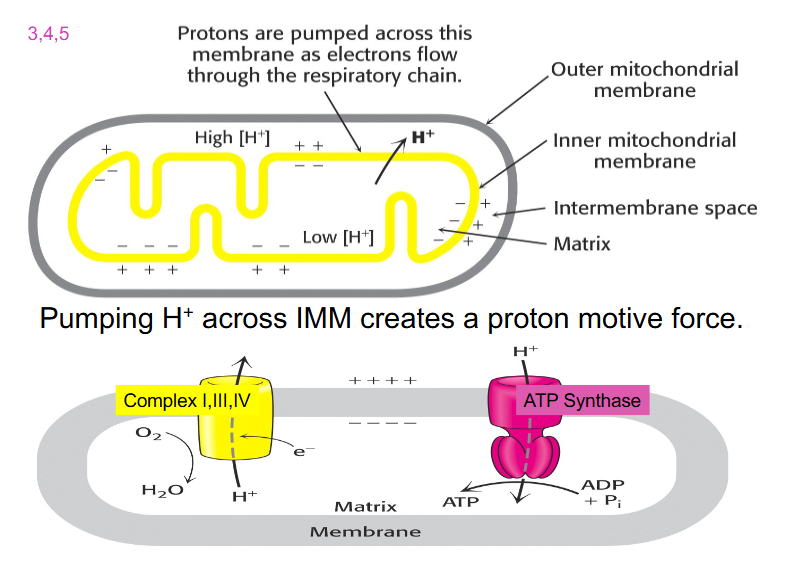
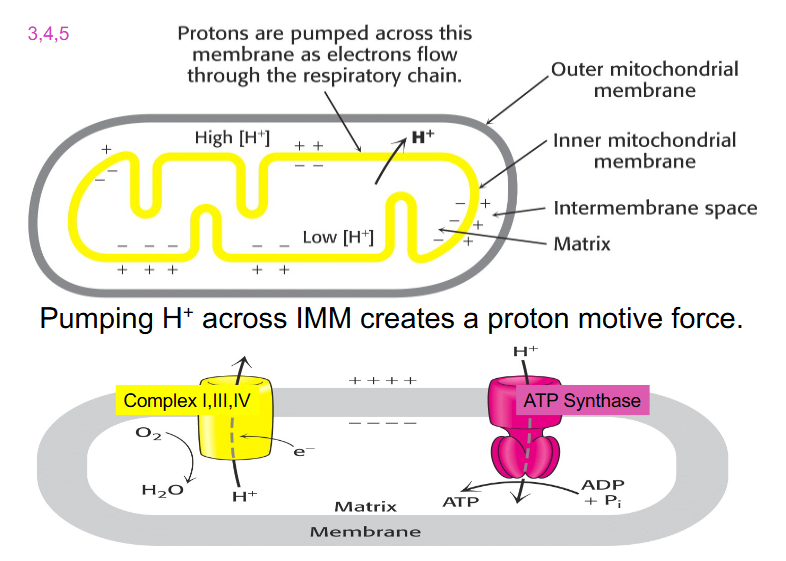
Pumping H+ across IMM creates a proton motive force
- outer mitochondrial membrane (OMM) in grey
- inner mitochondrial membrane (IMM) in yellow
- during ETC, NAD+ donates its electrons
- these electrons move down the ETC and during 3 complexes: 1, 3 and 4
- we get enough energy released in that electron movement to pump protons across the inner membrane to the intermembrane space
- creates a positive charge on one side of the membrane and therefore a more negative side on the matrix side
- also creates a chemical gradient because we've moved hydrogens across the membrane
- when enough protons are accumulated in the intermembrane space, they flow back through ATP Synthase
- when the protons move through, they physically turn the protein (changes shape)
- it binds to ADP and inorganic phosphate and converts it to ATP
.
- this will only happen if Oxygen accepts the electrons and is reduced to water (as the terminal acceptor)
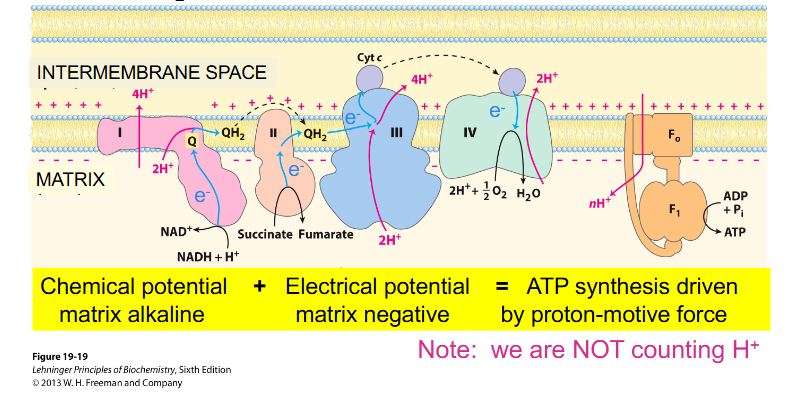
Oxidative Phosphorylation
- ETC Complexes pass protons from NADH/FADH2 to O2 (to make H2O)
- Simultaneously, H+ are pumped out of the matrix
- This H+ gradient (used to synthesise ATP) is a chemical and electrical gradient
- ubiquinone is shown to move around
- look at the diagram from left to right:
- we have electrons from NADH going into complex 1, then they move along the chain to a more positive carrier (as the numbers get bigger more positive, II, III, IV) (in terms of redox potential)
- Eventually, those electrons are used to reduce oxygen in the presence of protons to H2O (water)
- NADH donates its electrons to the first complex
- FADH2 (not on diagram) donates its electrons further along the chain
(rmbr that every NADH = 3 ATP, and FADH2 = 2 ATP)
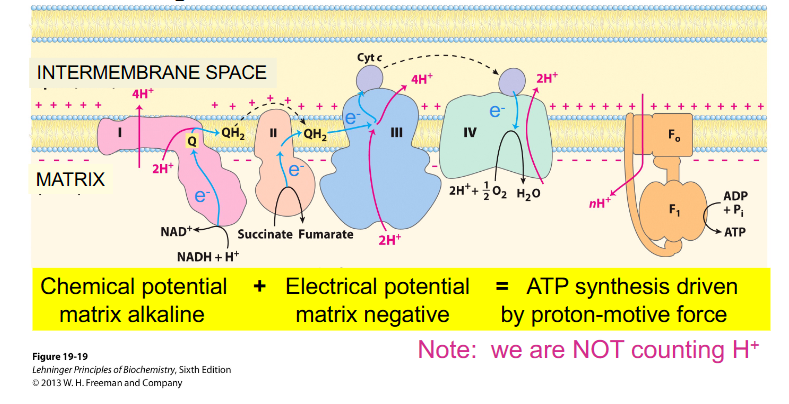
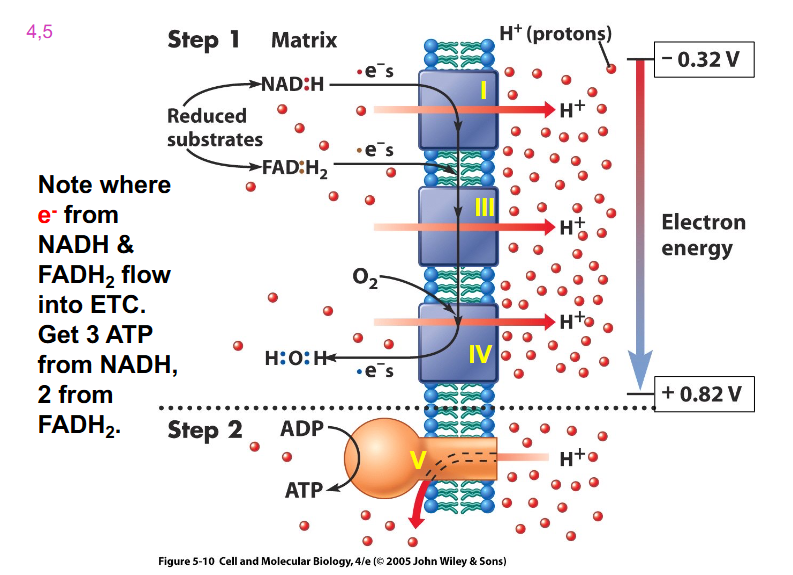
Electron Transport Chain Redox Potential
- look at Oxygen and NADH redox potential. See how if you move down that gradient (from -ve to +ve), we are getting successively more positive
- need to remember that complex 1, 3 and 4 are moving protons across
- NADH gives more ATP because protons move across and enable that first carrier to pump protons
- FADH2 misses the first carrier so it makes less ATP
- hence FADH2 has less protons move through the membrane and hence less protons move through ATP synthase
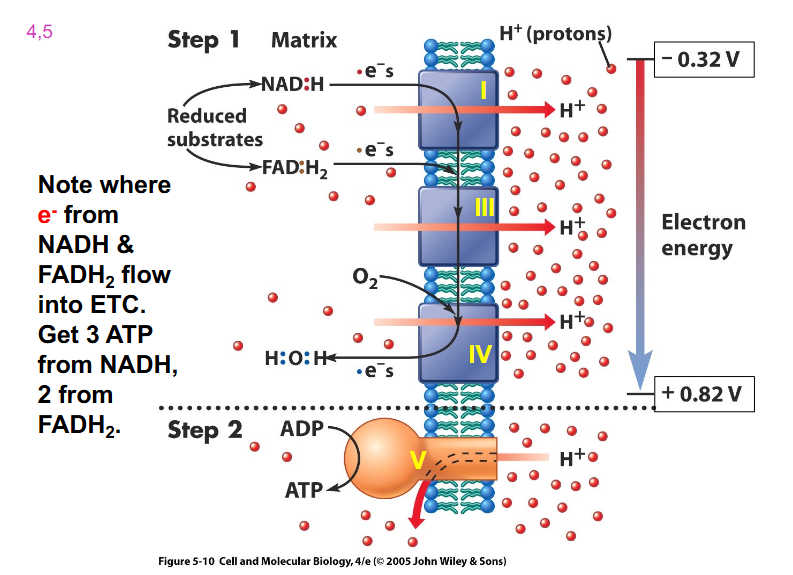
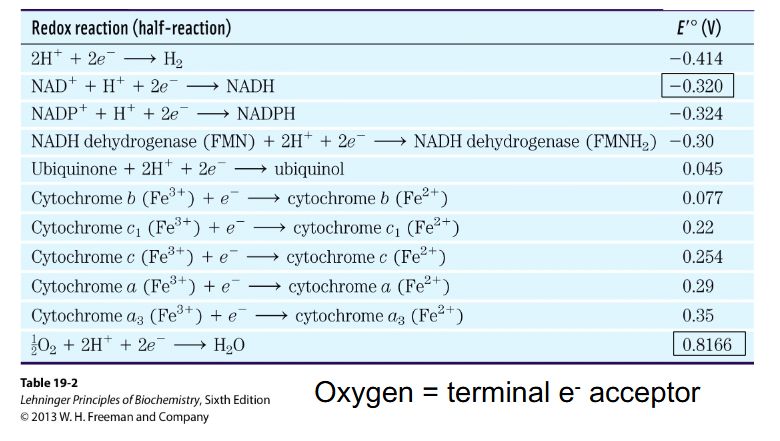
Electrons flow from more negative to more positive E'
- need to remember the redox potential of NAD+ reaction and production of water reaction (as circled)
- also need to remember that iron is an essential part of the electron transport chain
- Oxygen = terminal e- acceptor
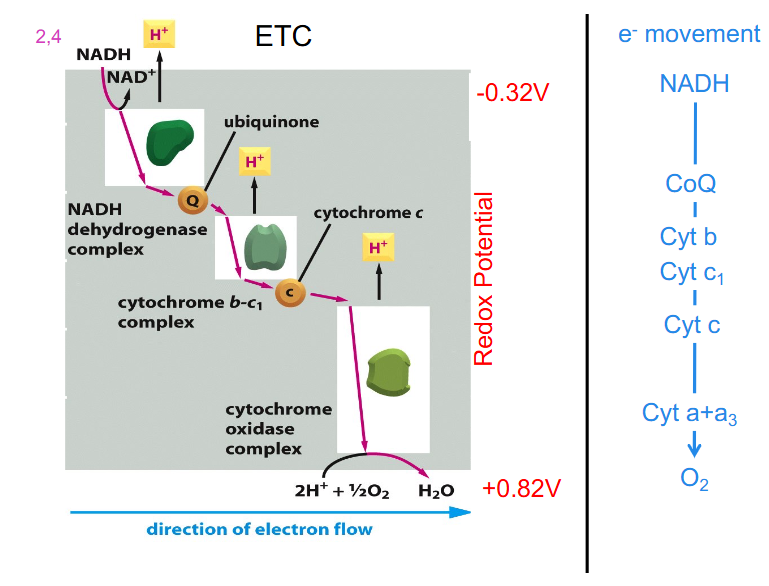
Summary of ETC So far
- electrons flow towards a more positive redox potential
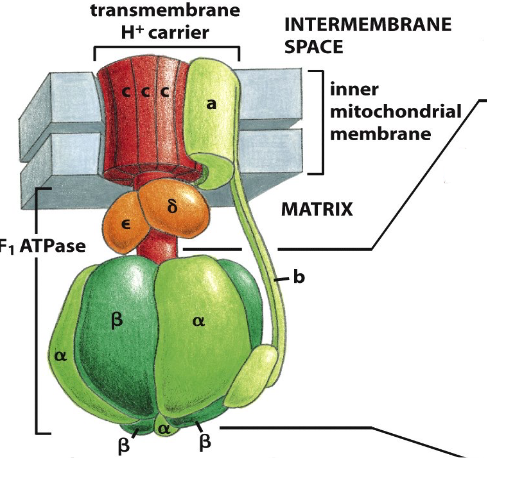
ATP Synthase (Complex V)
- a large transmembrane protein (multimeric) complex
- the electrochemical proton gradient across the inner membrane drives H+ back through ATP synthase
- Provides the energy to synthesise ATP from ADP + Pi in the matrix
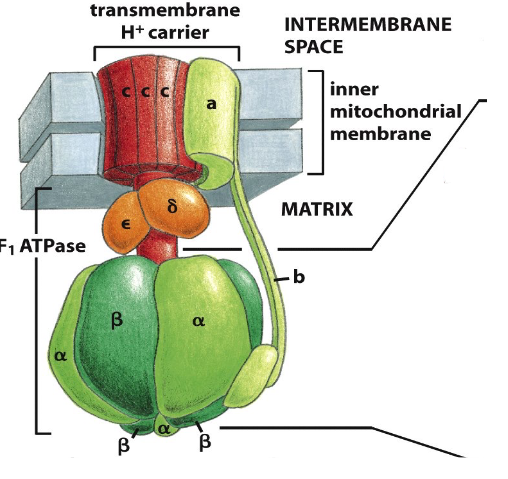
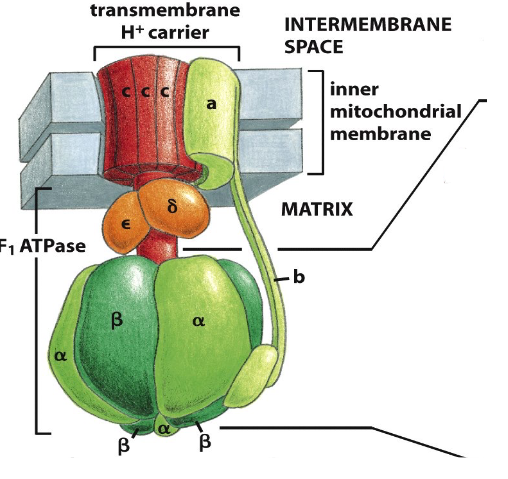
ATP Synthase: a multimeric molecular motor
- H+ flow through enzyme causing rotation of protein (shape change) allows ADP binding (substrate) and release of ATP (product)
- NADH and FADH2 are high energy electron carriers
- without mitochondria, they're not useful with mitochondria and all of the components of the electron transport chain as well as ATP synthase
Electron Transport Chain Movie
ON LMS
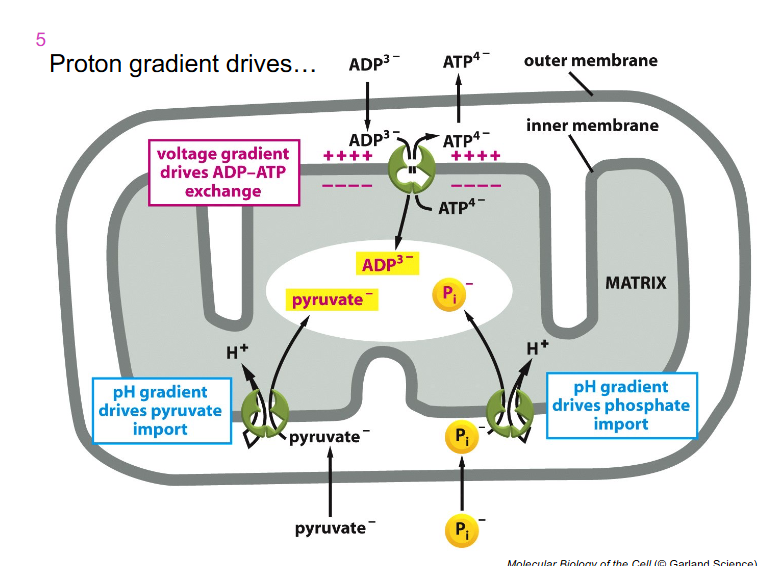
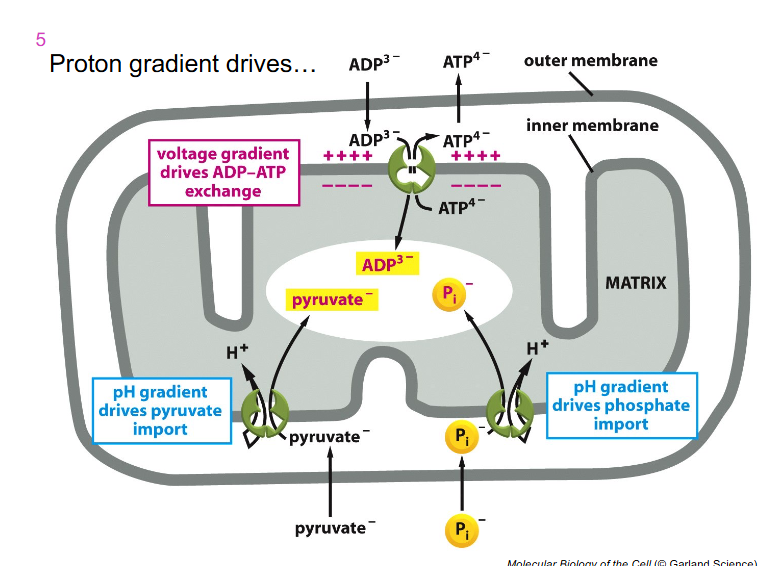
Proton Gradient Drives
- electron transport chain components are only expressed on the inner membrane
- lots of things can freely move across the outer membrane into the inter membrane space
- and then we have capacity with that gradient we form to allow ADP to be exchanged for ATP
- ADP can come into our inter membrane space as we generate that electrochemical gradient on the ETC
- so ADP can go inside the matrix and be used by the ATP synthase and then ATP can flow out into the rest of the cell
- inorganic phosphate can also move across the inner mitochondrial membranem because of that gradient we form
Cellular energy demands ______
control ETC
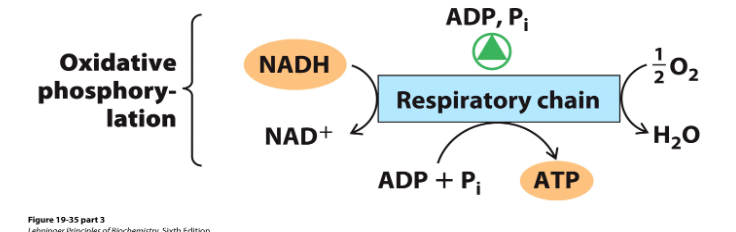
Regulating Oxidative Phosphorylation
- cellular energy demands dictate ETC
- intracellular [ADP] and ratio of [ATP] to [ADP][Pi]
- when the cell needs more ATP, the ETC is activated by high [ADP] and [Pi]
- Moreover, [ATP] and [ADP] set the rate of e- transfer through ETC via a series of coordinated controls on respiration, including glycolysis and TCA cycle
![<p>- cellular energy demands dictate ETC</p><p>- intracellular [ADP] and ratio of [ATP] to [ADP][Pi]</p><p>- when the cell needs more ATP, the ETC is activated by high [ADP] and [Pi]</p><p>- Moreover, [ATP] and [ADP] set the rate of e- transfer through ETC via a series of coordinated controls on respiration, including glycolysis and TCA cycle</p>](https://knowt-user-attachments.s3.amazonaws.com/1800a954-134e-4082-ace5-c26be0f23703.png)
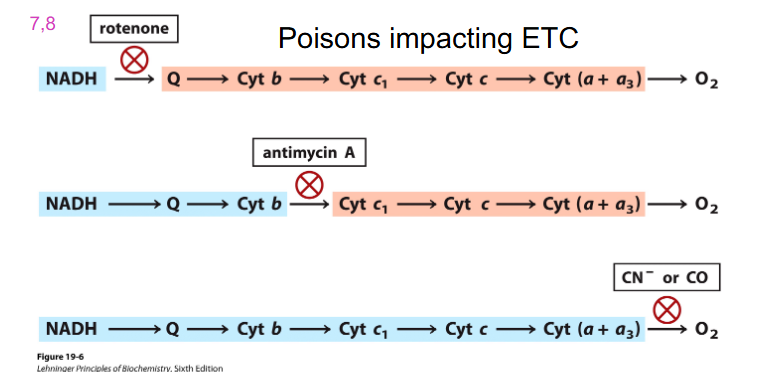
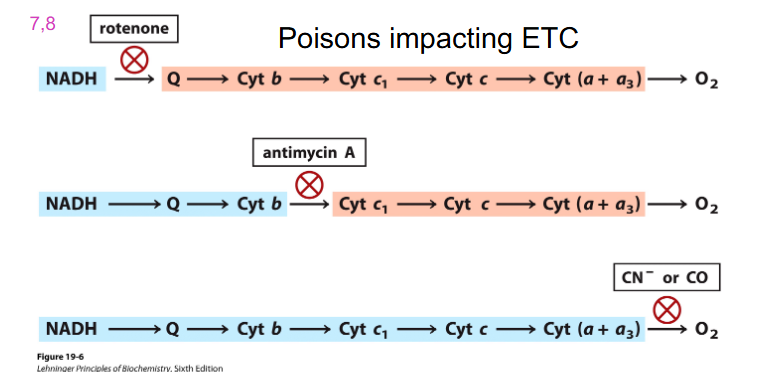
Poisons Impacting ETC
- Cyanide (CN) binds to Fe3+ of cytochrome oxidase complex (IV), terminal step of ETC, no further e- transport, no proton gradient formed
- no ATP produced via oxidative phosphorylation = death
- remember cyanide one not the others
- accumulation of NADH turns off TCA Cycle
- no ETC means that TCA Cycle turns off, hence Glycolysis turns off
- bsaically cyanide blocks at the terminal electron sector at the phase of oxygen
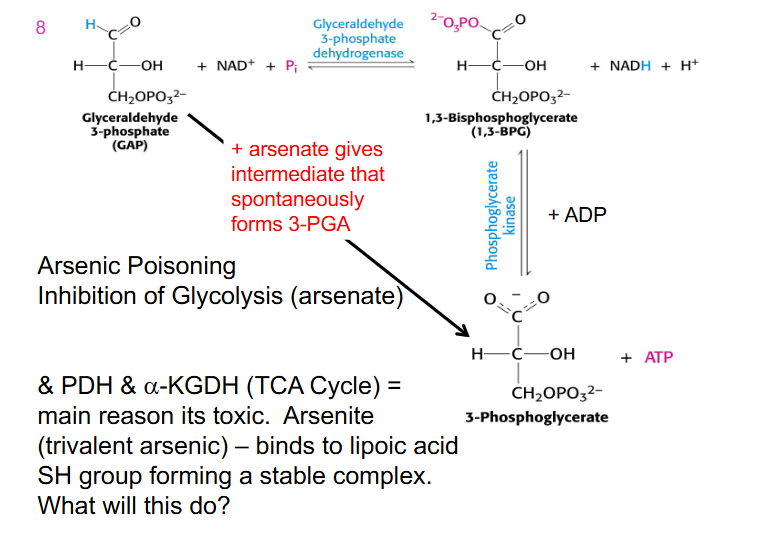
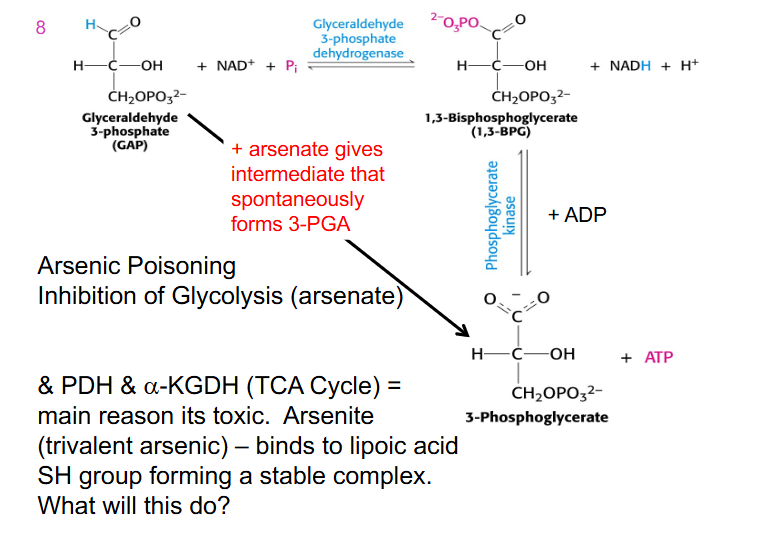
Arsenic Poisoning
- inhibition of glycolysis (arsenate)
- arsenate gives intermediate that spontaneously forms 3-phosphoglycerate
- no NADH is produced
- we wipe out energy generation in glycolysis
- arsenic inhibits enzymes that require lipoic acid
- if we turn off enzymes in the TCA cycle no energy is produced in mitochondria
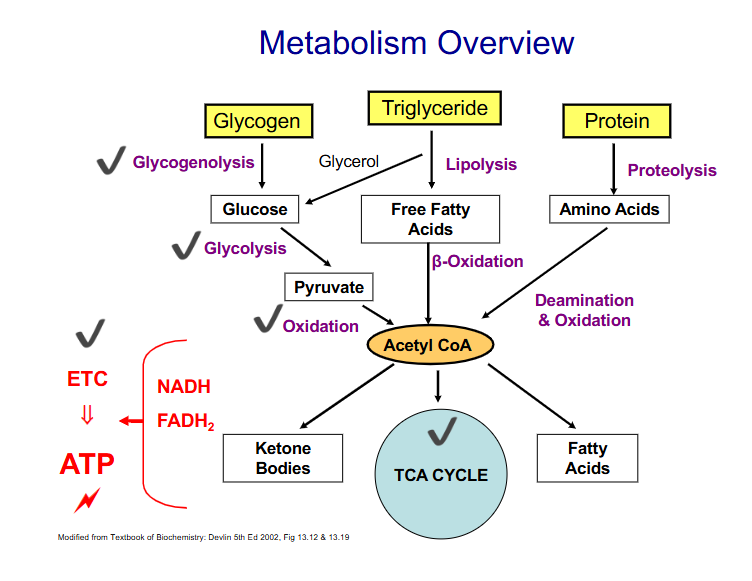
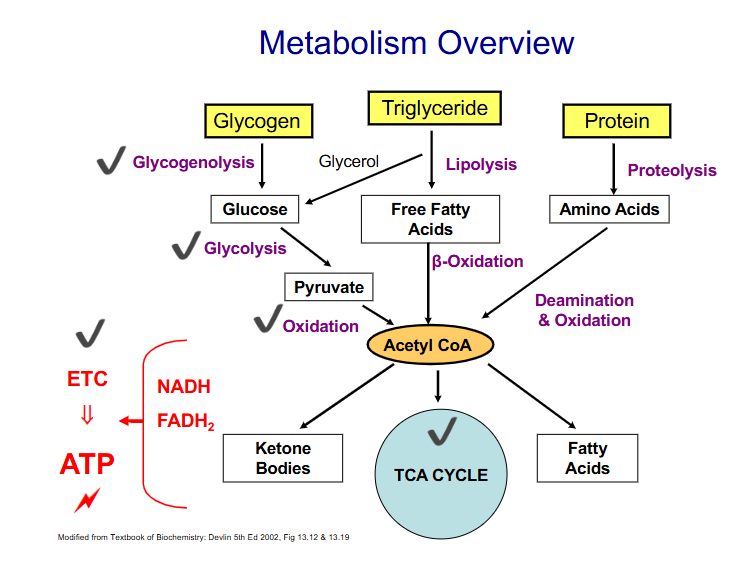
Summary of Metabolism
DIAGRAM ON SLIDE 22
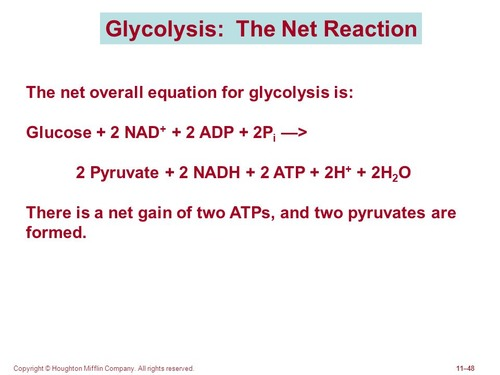
Glyocolysis overall reaction
Glucose + 2NAD+ + 2ADP + 2Pi → 2 Pyruvate + 2 NADH + 2ATP + 2H+ + 2H2O
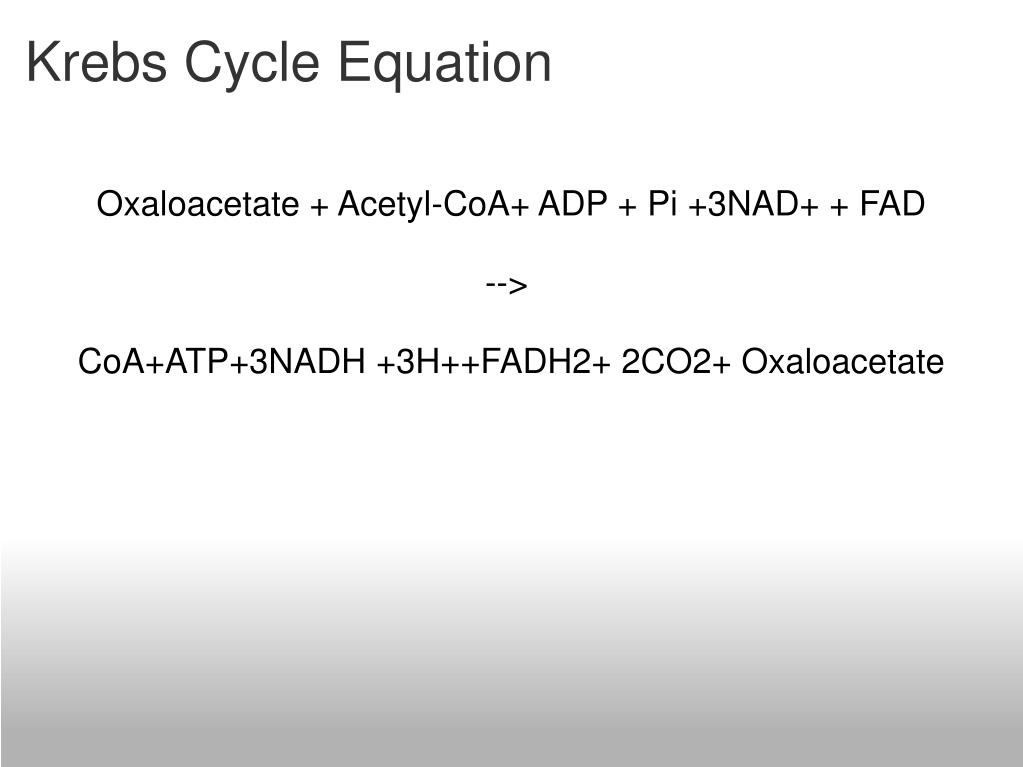
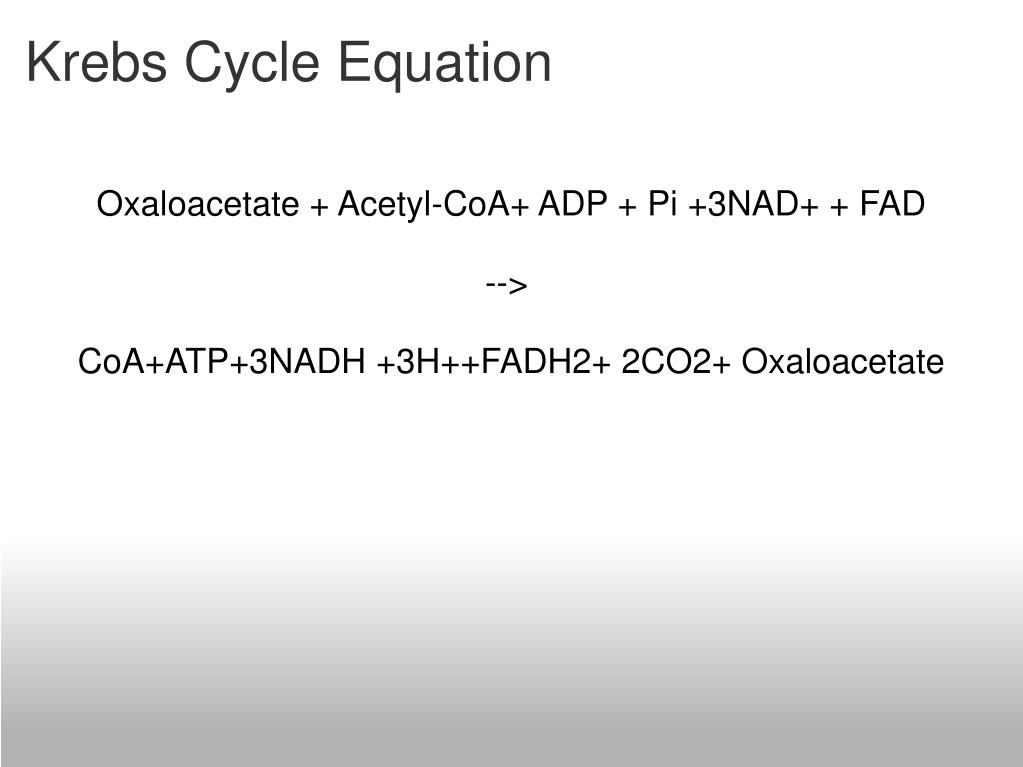
Krebs Cycle Overall Reaction
txet (1 FADH2 molecule produced in TCA, 3NADH produced)