Honors Chemistry Atoms Unit WCHS Mrs. Herrick
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Electron symbol, location in an atom, & electric charge
Symbol: e-
Location: Moving outside the nucleus
Electric charge: -
Proton symbol, location in an atom, & electric charge
Symbol: p
Location: Nucleus
Electric charge: +
Neutron symbol, location in an atom, & electric charge
Symbol: n
Location: Nucleus
Electric charge: N/A
Overall electric charge of an atom
Neutral— no overall electric charge
What values must be the same in the nucleus of an atom?
An atom must have as many electrons as there are protons
Atomic number (Z)
The number of protons in the nucleus of an atom
Mass Number (A)
The sum of the protons and neutrons in the nucleus
Isotope
An atom with the same number of protons, but a different number of neutrons— meaning that isotopes of the same element have the same atomic number but a different mass number
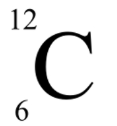
What type of notation is this?
Symbolic notation
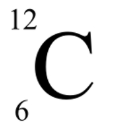
In this type of notation, what values go on the top and bottom?
Mass number (A) goes on the top, and atomic number (Z) goes on the bottom

What is this type of notation?
Hyphenated notation

In this type of notation, what is the value represented?
The mass number (A)
What are ions?
Atoms with unequal values of protons and electrons
In an ion, what value is changing?
The number of electrons— the atomic number (which is also the number of protons) does not change
What is a negative ion?
An ion where the atom gained electrons, making it negative, or an anion
What is a positive ion?
An ion where the atom lost electrons, making it positive, or a cation
What do two isotopes always have a different number of?
Mass number & the number of neutrons