Exothermic and endothermic reactions .1
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
energy is always…
conserved in chemical reactions,
so the total amount of energy in the universe at the end of a reaction is the same as it was before the reaction.
what are the two types of chemical reactions
exothermic reactions
endothermic reactions
what happens during a chemical reactions (to do with the surrounding)
energy is transferred to or from the surroundings
what is a exothermic reaction
energy is transferred to the surroundings
and the temperature of the surroundings increases
what are examples of exothermic chemical reactions
combustion reactions
many oxidation reactions
most neutralisation reactions
what are some every day uses where exothermic reactions occur
self-heating cans
hand warmers
what is an endothermic reaction
When energy is taken in from the surroundings
the temperature of the surroundings decreases.
what are some examples of endothermic reactions
thermal decomposition reactions
the reaction of citric acid and sodium hydrogencarbonate
what are some every day uses where endothermic reactions occur
instant ice packs which can be used to treat sports injuries.
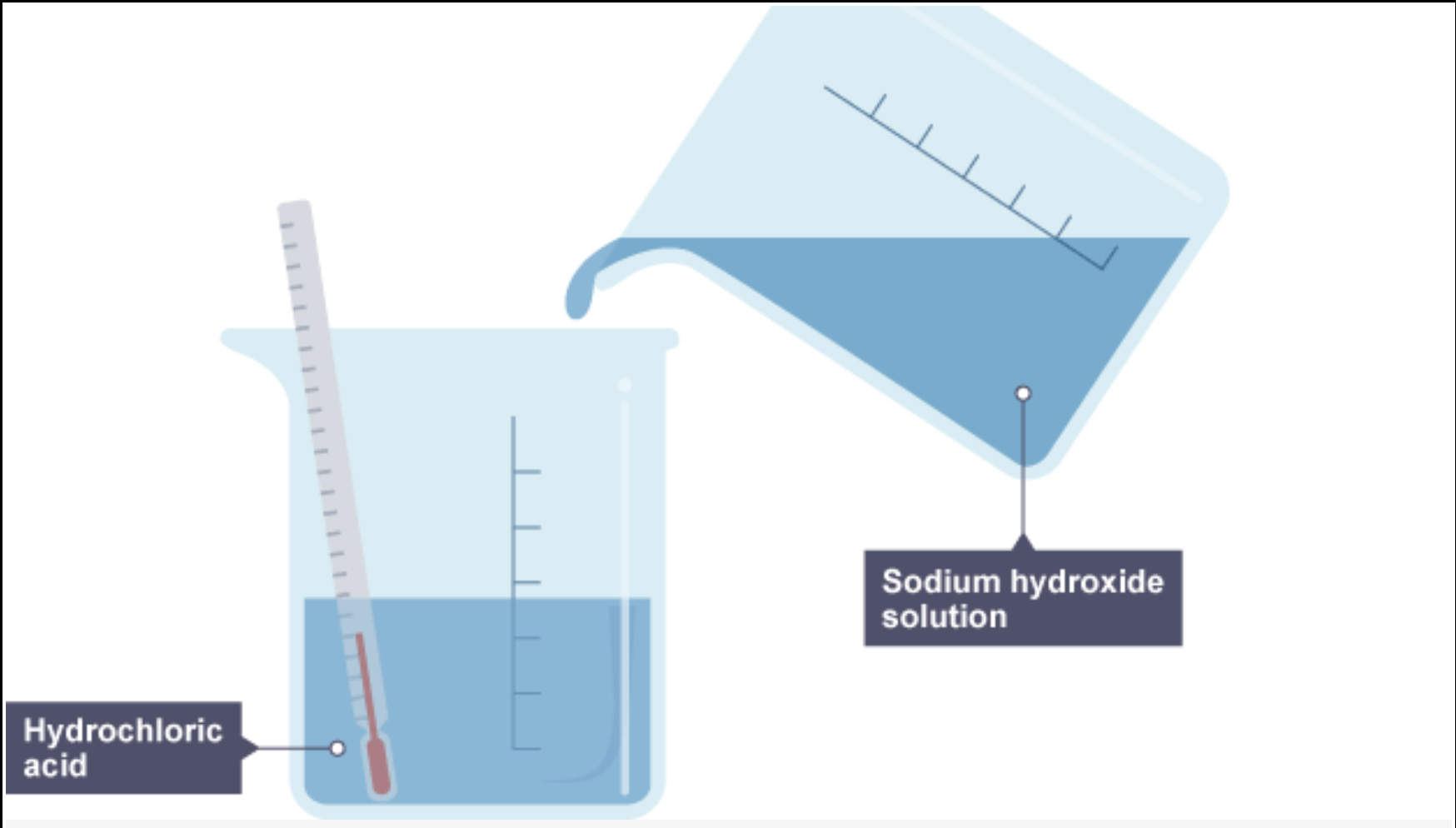
example of a exothermic reaction between dilute sodium hydroxide and hydrochloric acid
1. Sodium hydroxide solution is poured into a beaker of hydrochloric acid which contains a thermometer showing room temperature
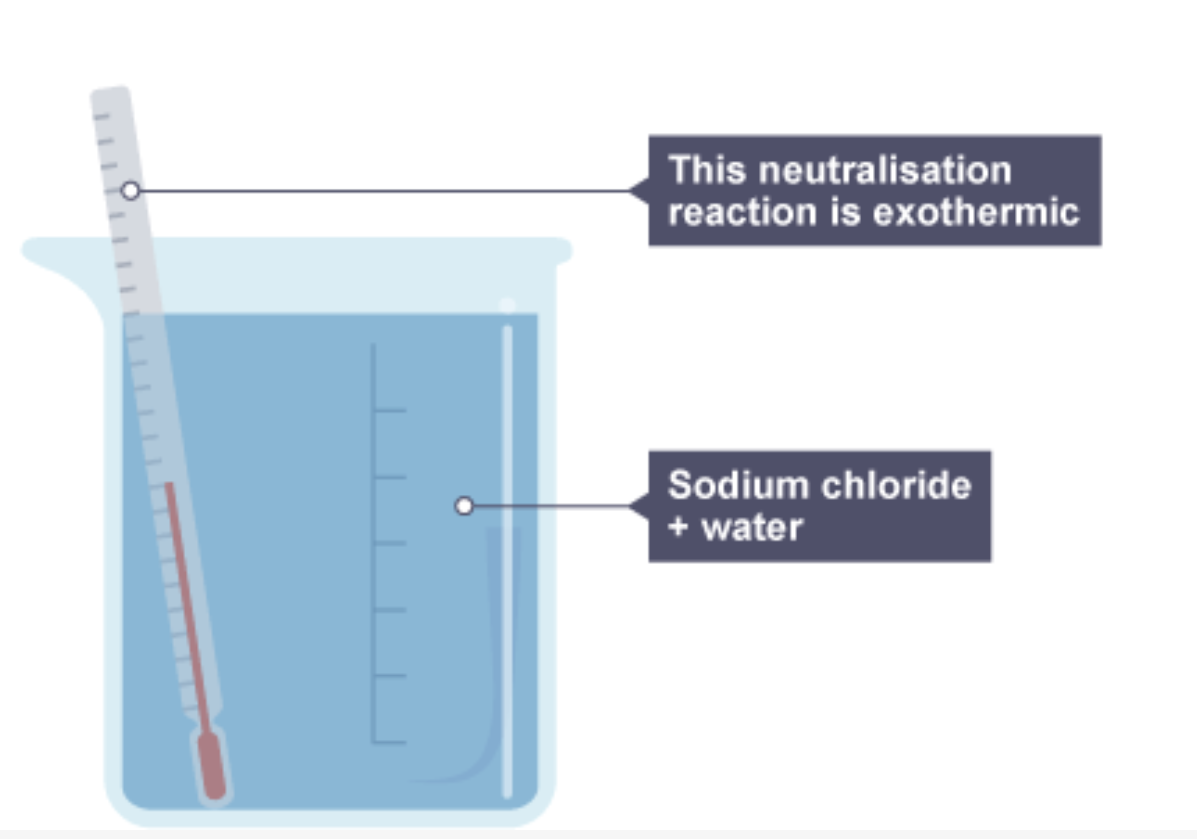
2. The beaker now contains sodium chloride and water, and the thermometer is showing a rise in temperature, so the neutralisation reaction is exothermic
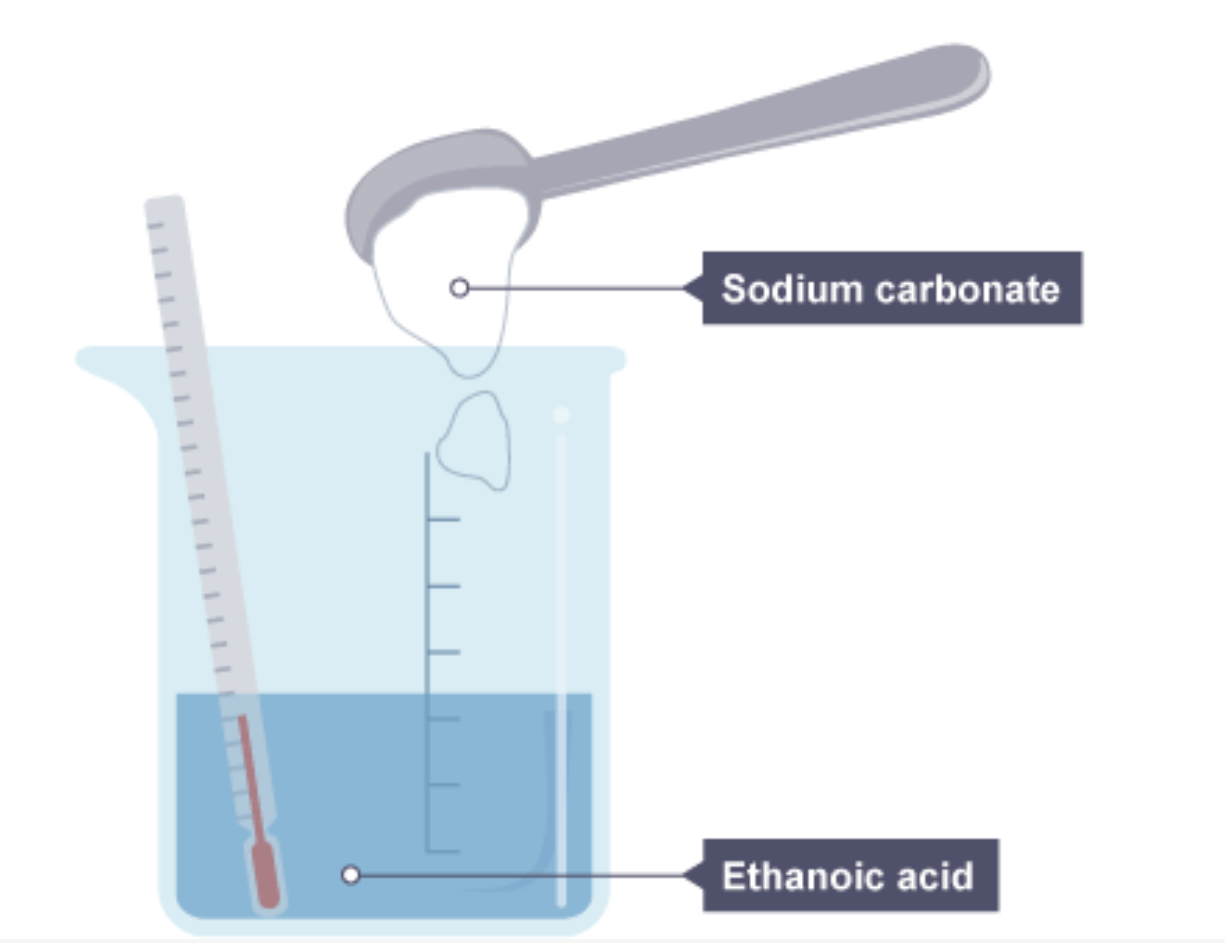
example of a endothermic reaction between sodium carbonate and ethanoic acid
Sodium carbonate powder is tipped into a beaker of ethanoic acid which contains a thermometer showing room temperature
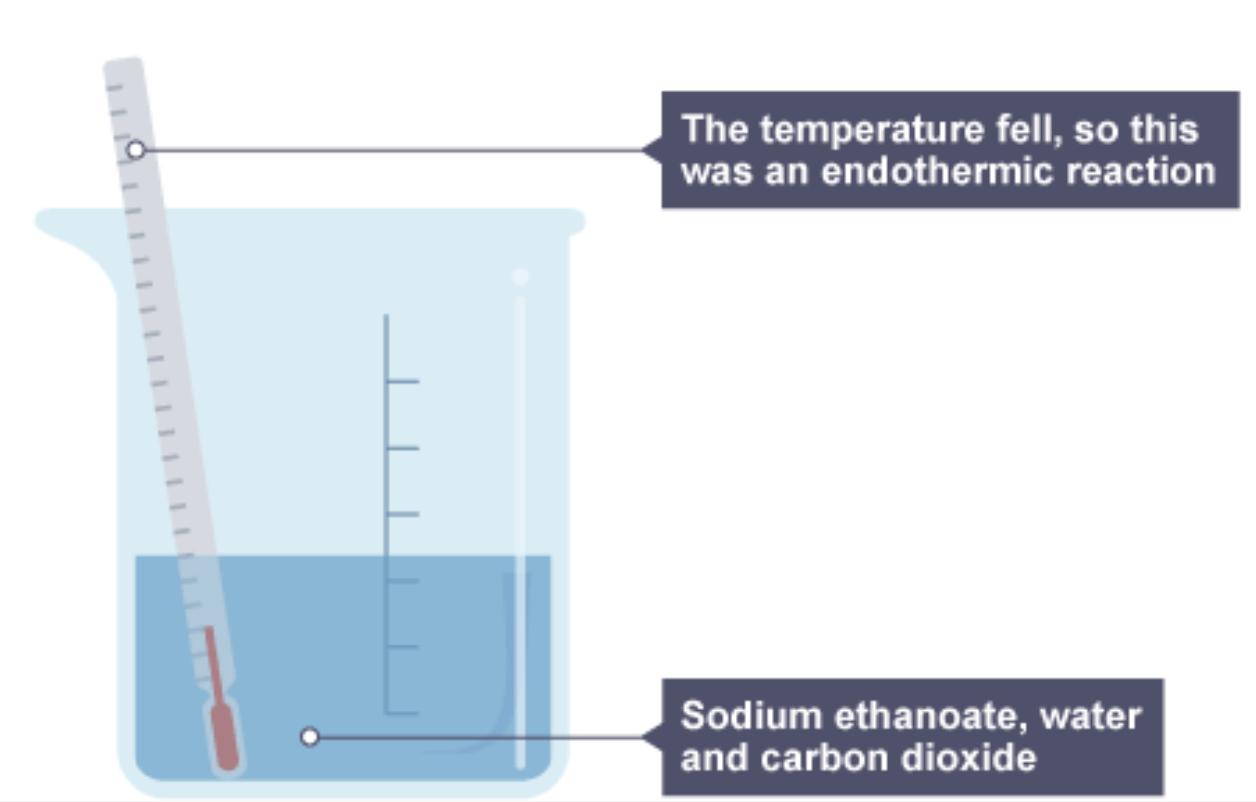
The beaker now contains sodium ethanoate, water and carbon dioxide, and the thermometer is showing a fall in temperature, so this was an endothermic reaction
In endothermic reactions….
energy enters
In exothermic reactions…
energy exits.
Required practical - temperature changes in reacting solutions
Reacting two solutions, eg acid and alkali
Reacting two solutions, eg acid and alkali
Place the polystyrene cup inside the glass beaker to make it more stable.
Measure an appropriate volume of each liquid, eg 25 cm3.
Place one of the liquids in a polystyrene cup.
Record the temperature of the solution.
Add the second solution and record the highest or lowest temperature obtained.
Change your independent variable and repeat the experiment. Your independent variable could be the concentration of one of the reactants, or the type of acid/alkali being used, or the type of metal/metal carbonate being used.
Required practical - temperature changes in reacting solutions
Reacting a solid with a solution, eg metal and acid
Reacting a solid with a solution, eg metal and acid
Place the polystyrene cup inside the glass beaker to make it more stable.
Measure an appropriate volume of the solution, eg 25 cm3.
Measure an appropriate mass of the solid, or select a suitable sized piece of metal.
Place the solution in a polystyrene cup.
Record the temperature of the solution.
Add the solid and record the highest or lowest temperature obtained.
Change your independent variable and repeat the experiment. Your independent variable could be the surface area of the solid, or the type of acid being used, or the type of metal being used.
Required practical - temperature changes in reacting solutions
analysis
The bigger the temperature change in the reaction, the more energy is absorbed or released
Required practical - temperature changes in reacting solutions
evaluation
The biggest source of error in this experiment is unwanted heat transfer. Using a lid can help to reduce this.
Required practical - temperature changes in reacting solutions
2 hazards
Dilute acids and alkalis
Solutions of metal salts (used in displacement reactions)
Required practical - temperature changes in reacting solutions
possible harm + precaution of Dilute acids and alkalis
May irritate the skin or eyes
Avoid contact with skin, rinse off skin if necessary, wear eye protection
Required practical - temperature changes in reacting solutions
possible harm + precaution of Solutions of metal salts (used in displacement reactions)
Dangerous to the environment
Dispose of metal salt solutions as advised by teacher, some metal salts are collected for safe disposal rather than being poured down the normal drain
what is a energy level diagram
shows whether a reaction is exothermic or endothermic
It shows the energy in the reactants and products
and the difference in energy between them.
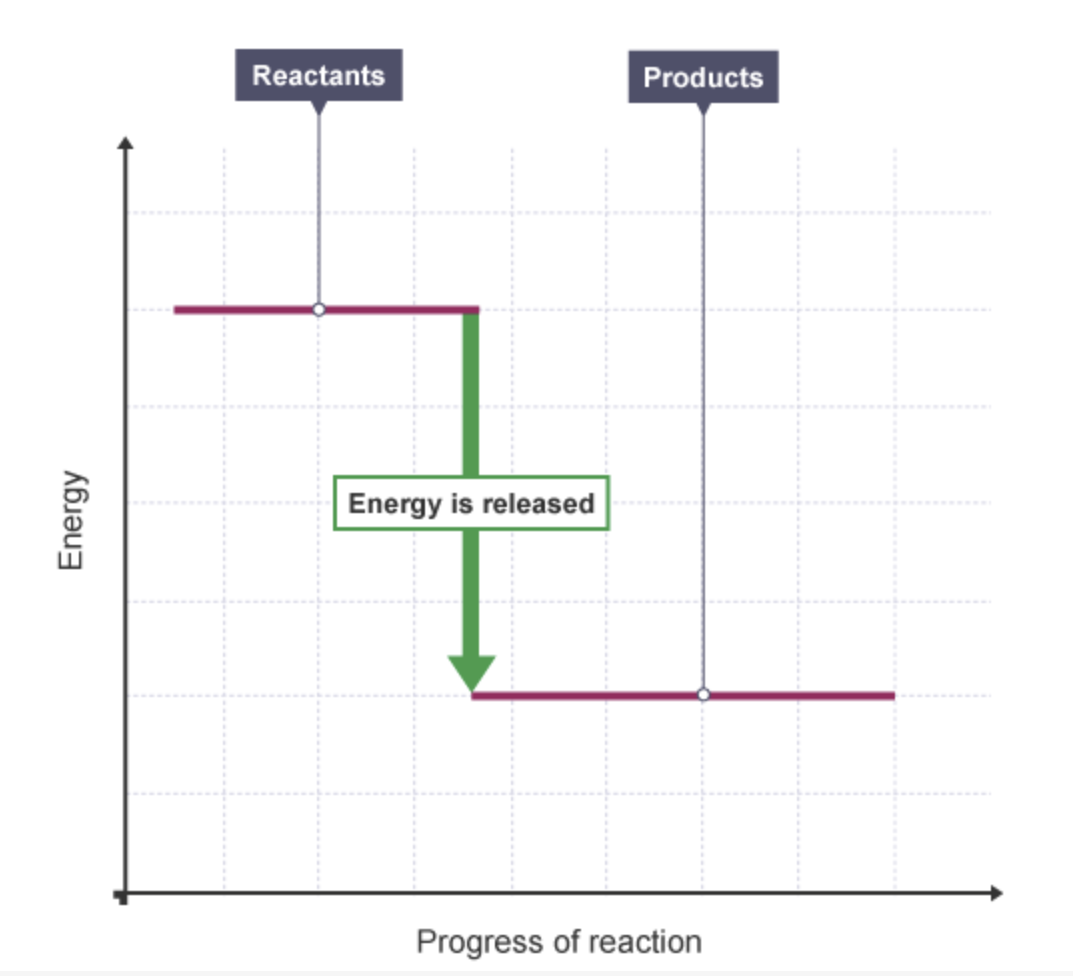
what happens to the energy level in an exothermic reaction + why
decreases
because energy is given out to the surroundings.
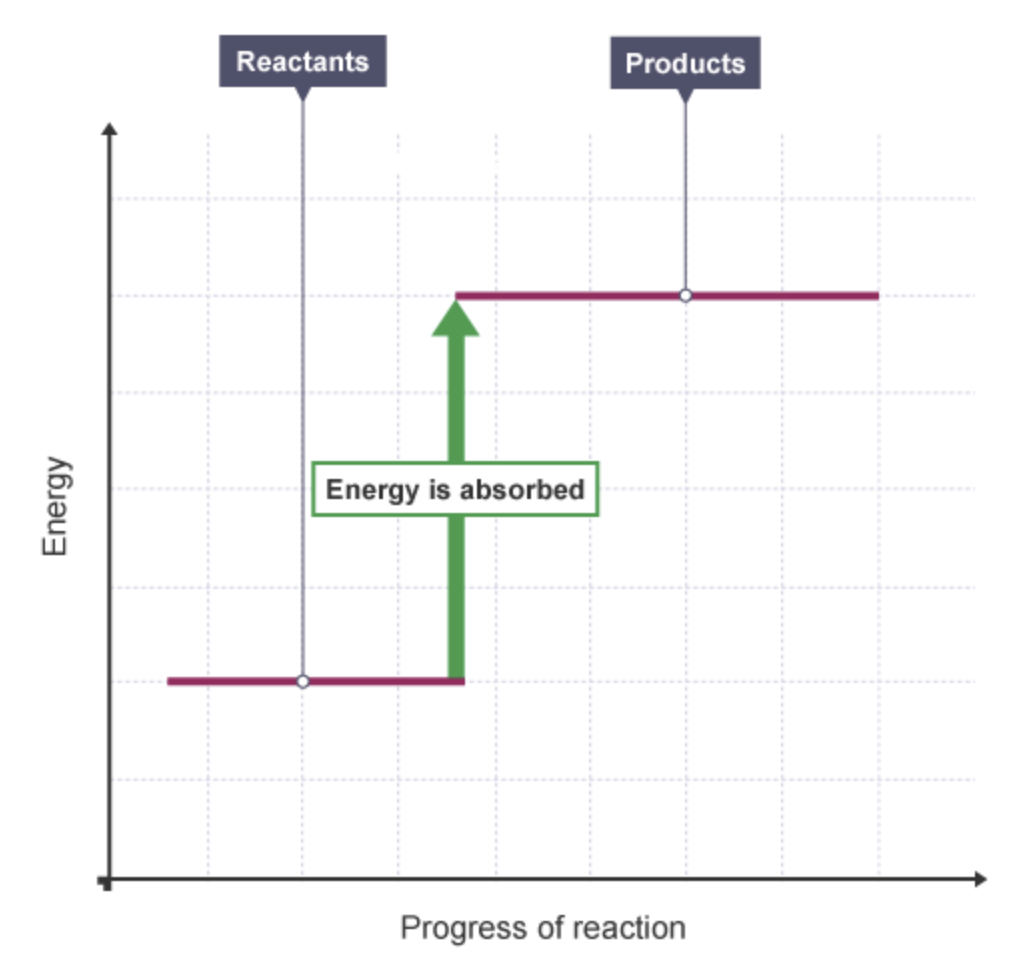
what happens to the energy level in an endothermic reaction + why
increases
because energy is taken in from the surroundings.
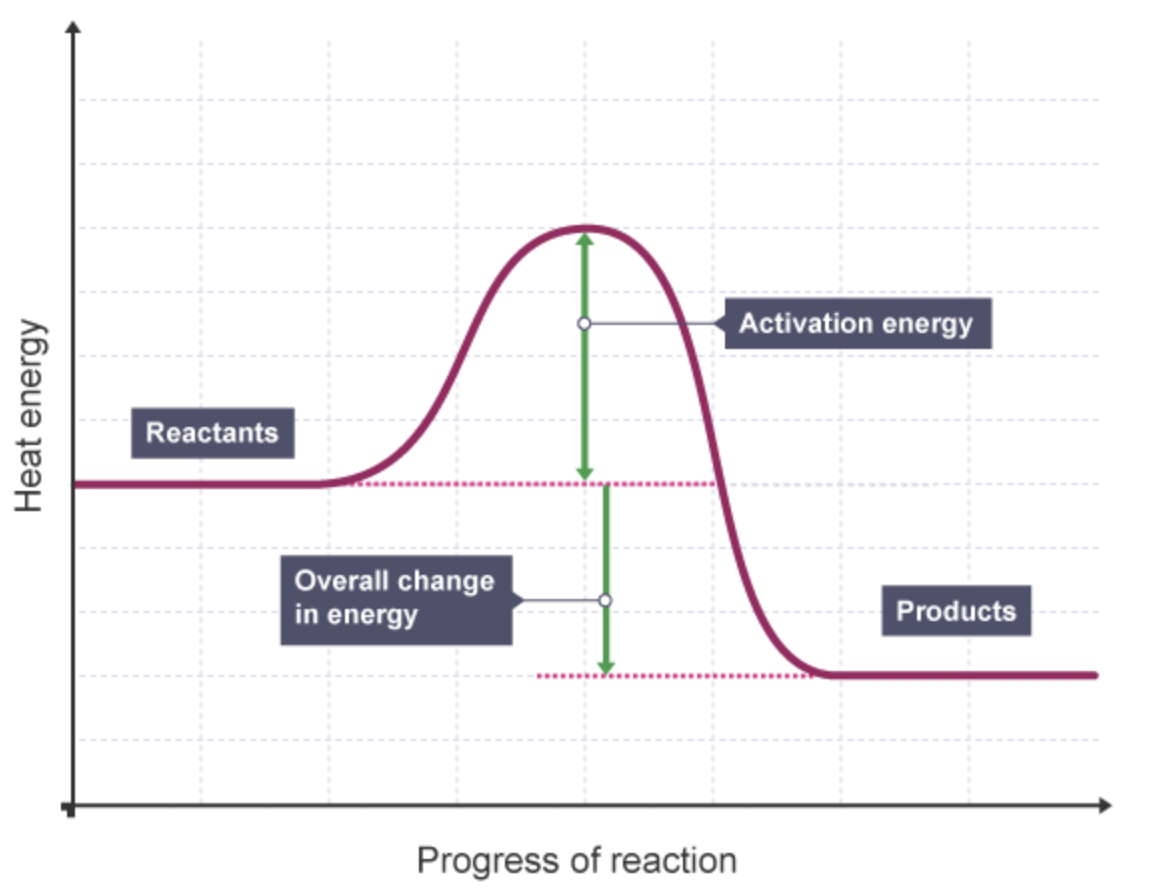
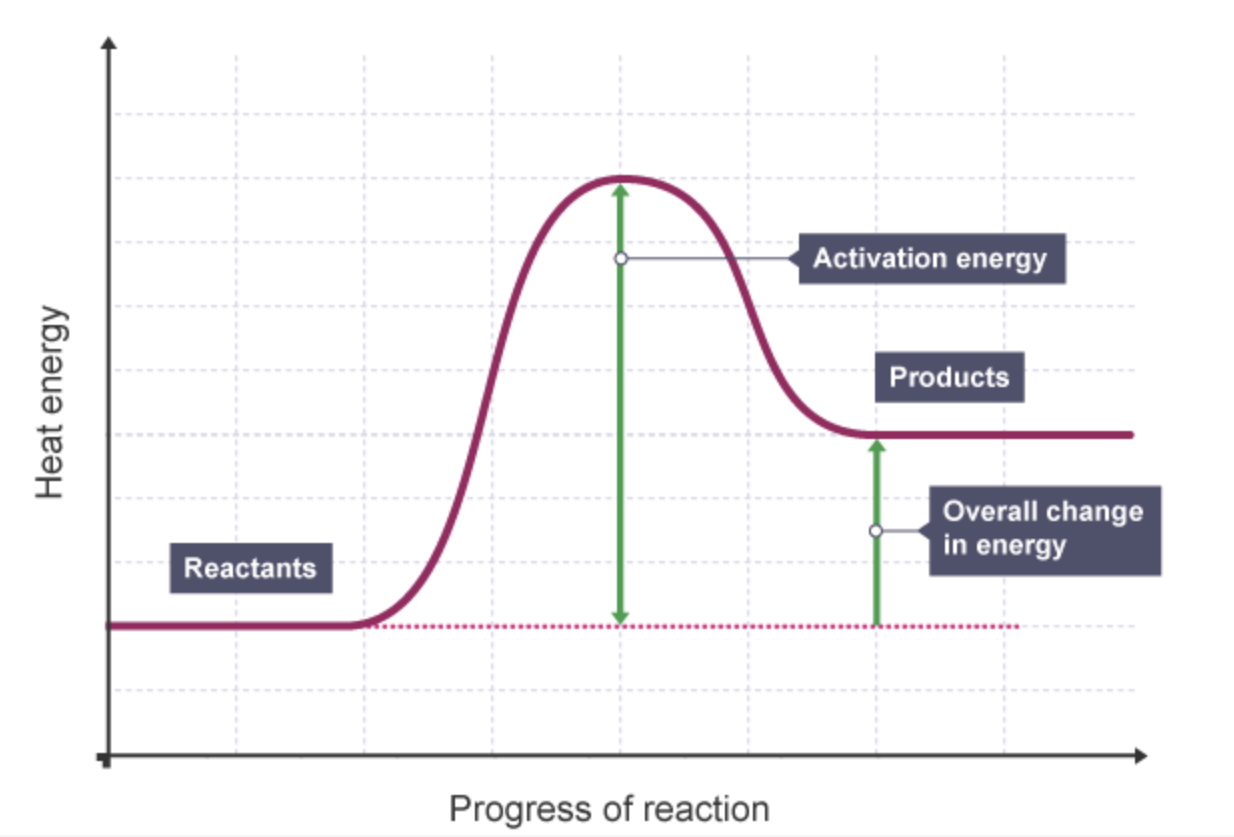
what is the activation energy in a reaction profile
the minimum energy needed by particles when they collide for a reaction to occur
what is the overall change in energy in a reaction
the difference between the energy of the reactants and products.
what is the reaction profile for an exothermic reaction.
what is the reaction profile for an endothermic reaction
how is energy transferred
when bonds are broken or are formed
what does an endothermic reaction do to bonds
breaking bonds
takes energy in
what does an exothermic reaction do to bonds
building bonds
gives energy out
what happens during a chemical reaction to the reactants and products
During a chemical reaction:
bonds in the reactants are broken
new bonds are made in the products
in an exothermic reaction where is more heat energy needed (making or breaking bonds)
in a exothermic more heat energy is released in making bonds in the products
than is taken in when breaking bonds in the reactants
in an endothermic reaction where is more heat energy needed (making or breaking bonds)
in a endothermic reaction more heat energy is taken in when breaking bonds in the reactants
than is released in making bonds in the products
how can the energy change in a reaction be calculated
using bond energies
what is a bond energy
A bond energy is the amount of energy needed to break one mole of a particular covalent bond.
how do you calculate the energy taken in (bond energy)
add together the bond energies for all the bonds in the reactants - this is the 'energy in'
hwo do you calculate the energy given out (bond energy)
add together the bond energies for all the bonds in the products - this is the 'energy out'
how do you calculate the energy change
energy change = energy in - energy out
if the energy change is negative what is the reaction
exothermic
if the energy change is positive what is the reaction
endothermic