Review 3 - Isotopes & Average Atomic Mass
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
isotope
Atom with the same number of protons but different number of neutrons compared to other atoms of the same element.
2
New cards
symbol notation
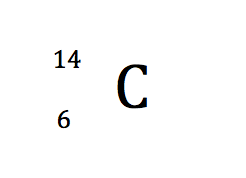
3
New cards
atomic mass number
number of protons + number of neutrons
4
New cards
atomic number
number of protons
5
New cards
word notation
carbon-6
6
New cards
average atomic mass
weighted average of all isotopes of that element
7
New cards
average atomic mass formula
(mass x %abundance) + (mass x %abundance) + …