[ 2FINAL ] Chemistry - Kinetic Molecular Theory
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Temperature
the measure of the average kinetic energy (KE) of the molecules
Pressure
the force exerted per area
Air Pressure
force exerted on the walls of the container upon collision
Barometer
an instrument used to measure atmospheric pressure. it helps determine whether air pressure is increasing, decreasing, or remaining stable.
273.15
What do you add to Celsius to turn it into Kelvin?
Boyle’s Law
What is the name of this formula?
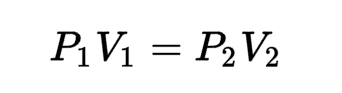
Charles’s Law
What is the name of this formula?
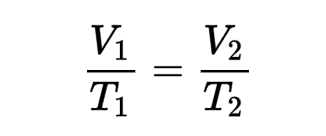
Gay-Lussac’s Law
What is the name of this formula?
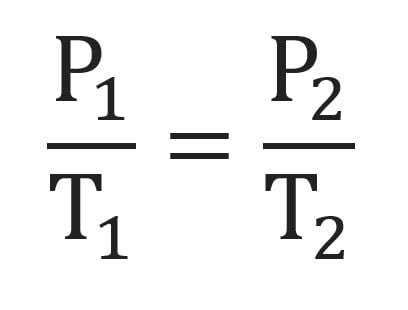
Temperature
If this is constant, pressure is inversely proportional with volume
Pressure
If this is constant, volume is directly proportional with temperature
Volume
If this is constant, pressure is directly proportional with temperature
Decreases
If temperature is constant, when volume increases, pressure _____
Increases
If pressure is constant, when temperature increases, volume _____
Increases
If volume is constant, when pressure increases, temperature _____
Air Pressure Gauge
This measures the pressure of air in a pneumatic system.