MTLB - 32 sections, 5527c
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Section 1 – Title
Philippine Medical Technology Act of 1969
Section 2 – Definition of terms
Medical Technology – Auxiliary branch of laboratory medicine which deals with the examination.
Pathologist – A duly registered physician trained in laboratory medicine.
Medical Technologist (RMT) – Graduate of Bachelor of Science in Medical Technology/Hygiene, licensed under this Act.
Medical Technician – Not a BSMT graduate, but passed the Civil Service Exam, and works under an RMT or Pathologist.
Accredited Medical Technology Training Laboratory – Approved by the Department of Health (DOH).
Recognized School of Medical Technology – Approved by the Department of Education (DepEd) upon Council of Medical Technology Education recommendation.
Council – Refers to Council of Medical Technology Education.
Board – Refers to Board of Examiners for Medical Technology.
Section 3 – Council of Medical Technology Education, It’s composition
Chairman – Secretary of Education or Director of Private Education
Vice Chairman – Director of the Bureau of Research and Laboratories of the Department of Health
Members:
Chairman of the Board of Medical Technology
Two Members of the Board
Dean, Institute of Hygiene, University of the Philippines (UP)
Representative of Deans/Heads of Private MedTech Schools
President of PAMET
President of the Philippine Society of Pathologists (PSP)
Section 4 – Compensation and Traveling Expenses of Council Members
₱25/meeting (max 2/month)
Government officials: No per diem if already receiving salaries
The Chairman and Members of the council shall be entitled to travel expenses related to official duty
Section 5 – Functions of the Council of Medical Technology Education
(Mnemonic: RIPort CARRD)
R – Recommend minimum required. curriculum
I – Inspect medical technology schools in the
country to ensure the maintenance of high
standard
P – Promulgate and prescribe and enforce
necessary rules and regulations for the proper
implementation of the foregoing functions.
C – Certify admission for internship and Collect
5 pesos
A – Approve medical technology schools &
recommend closure of substandard schools
R – Require all medical technology schools to
submit an annual report
R – Recommend approval of refresher course
D – Determine and prescribe the number of
students to be allowed to take up the medical
technology course in each school
Section 6 – Minimum required course
4-year BSMT/BSH + 12-month internship
The council is authorized to change, remove or add subjects listed as the needs and demands
Secretary of Education may approve curriculum updates
Section 7 – Board of Examiners of Medical Technology
Chairman – A Pathologist
Two Members – Licensed Medical Technologists (RMTs)
Appointed by the President of the Philippines from PAMET and PSP
Note: No member shall be allowed to be reappointed more than one
The President of the Philippines shall fill the vacancy that may occur, but the appointee shall serve only the unexpired term of the incapacitated member.
Section 8 – Qualifications of Examiners
Filipino citizen
Good moral character
RMT or Qualified Pathologist with BSMT/BSH
At least 10 years in Laboratory Practice or Medical Technology
Not a faculty member or investor in any MedTech school, or have any pecuniary interest, direct or indirect, in such institution
But for the first three years following the approval of this Act, the requirement mentioned in number 4 shall be reduced to 5 years.
Section 9 – Executive Officer of the Board
Commissioner of Civil Service (now Professional Regulation Commission – PRC)
The Secretary of the Board Examiners - shall also be the Secretary of the Board.
He shall keep a register of all persons to whom certificates of registration have been granted.
Section 10 – Compensation of Members of the Board examiners of the medical technology
₱10.00 per applicant examined
₱5.00 per each applicant granted a certificate of registration without exam
Section 11 – Functions and duties of the Board
Administer the Act
Administer oaths
Issue/suspend/revoke certificates
Investigate violations
Issue subpoena and subpoena duces tecum
Draft necessary rules
Section 12 – Removal of Board members
Can be removed by the President of the Philippines
Temporary replacement if suspended
By what ground can a member be removed?
Neglect of duty
Incompetency
Malpractice or unprofessional
Unethical
Immoral or dishonorable conduct after having been given opportunity to defend himself in a proper administrative investigation
Section 13 – Accreditation of schools of Medical Technology and training labsoratories
Schools: Approved by Department of Education
Labs: Approved by DOH, based on Council of Medical Technology recommendation
Section 14 – Inhibition against the Practice of Medical Technology
Exemptions from license:
Registered physicians
Foreigners (consultants, visiting professors)
U.S. Armed Forces MedTechs in PH
Section 15 – Examination
Frequency: Once a year (Annual)
Locations: Manila, Cebu, Davao
Month: August/September
Notices should be published in 3 national newspapers by the Secretary of the Board at least 30 days of the examination.
Section 16 – Qualifications of examination
Good health and moral character
Graduate of recognized BSMT/BSH
Non-MT grads: 5 years practice before June 21, 1969 + internship
(Prior to the enactment of this Act (June 21, 1969) that they meet the maximum requirement mentioned in SEC. 6 exceeding 1 year undergrad internship or practical training)
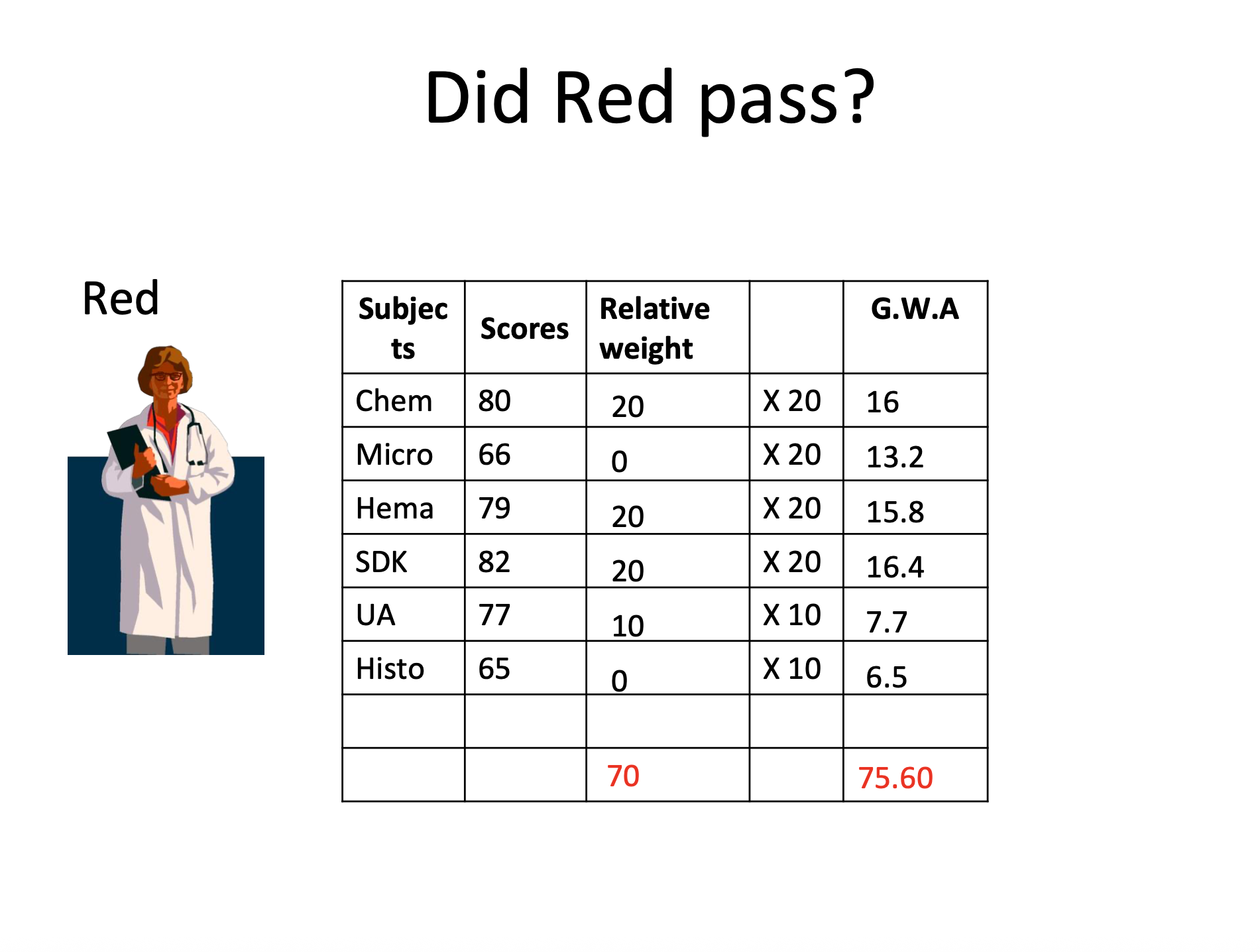
Section 17 – Scope of Examination
Subject | % Weight |
|---|---|
Clinical Chemistry | 20% |
Microbiology & Parasitology | 20% |
Hematology | 20% |
Blood Banking & Serology | 20% |
Clinical Microscopy | 10% |
Histopathologic Techniques | 10% |
= 100%
→ BOARD - Prepare the schedule of subjects
→ Submission to the COMMISSIONER OF CIVIL SERVICE for publication
(4 months before exam)
→ The board should compute the general average according to the relative weight of the subject
→ The board may change, add or remove from the list of subjects or weight above
→ Prior approval to the council
Section 18 – Report of rating
Board of Examiners reports to Commissioner of Civil Service (now PRC) → 120 days after exam
Results submitted to President of the Philippines for approval
Section 19 – Rating in the examination
Passing average: 75%
No major subject below 50%
Did not fail 60% of relative weight
→ If you fail, you can repeat up to: 3 years
TO BE ABLE TO TAKE THE 4TH EXAM, YOU SHOULD HAVE:
12 months refresher course in accredited SMT
12 months post-grad training in an accredited laboratory
IF NON-BSMT/NON-BSH failed for the 3rd time in board exam, WILL NOT BE ALLOWED TO TAKE THE BOARD EXAM ANYMORE
Section 20 – Oath-taking
All passers must take a Professional Oath before the board or practicing Medical Technology
Section 21 – Issuance of certificate of registration
≥ 21 years old
Passed the board exam
ORWith BSMT/BSH and 3+ years lab experience
Non-BSMT/BSH but medical field grads with 5+ years lab work
Section 22 – Fees
Service | Fee |
|---|---|
Exam & Registration | ₱50 |
Certificate of Registration without Exam | ₱25 |
Replacement of Certificate (Lost, Destroyed, or mutilated) | ₱10 |
Section 23 – Refusal to issue certificate
The Board shall refuse to issue a certificate of registration to any person:
Immoral or Dishonorable conduct
Unsound mind, or
Incurable communicable disease
The board shall give to the applicant a written statement setting forth the reason for its action
Section 24 – Administrative investigation or suspension of certificate
Has done anything that as mentioned in SEC. 29
Requires hearing by 2 Board Members + Legal Officer
Public hearing will be conducted, The MT and witnesses will be cross- examined, and the MT may be represented by a lawyer or themself
The board may:
Reprimand the MT (issue a formal warning) = 2/3
Suspend the certificate (maximum of 2 years) - should be a majority vote = 2/3
Revoke the certificate (permanent cancellation) - should have unanimous vote of the 3 members of the board = 3/3
After the decision:
If revoked or suspended, the MT must:
Surrender their Certificate of Registration
Within 30 days of final decision
Suspension period starts only after the certificate is surrendered
Section 25 – Appeal
Appeal to Civil Service Commissioner (now PRC)
The decision will become final 30 Days after the suspension/revocation
UNLESS, the respondent has appealed to the President of the Philippines within 30 days
Section 26 – Reinstatement, reissue or replacement of certificate
The suspension will automatically be lifted on the expiration of the suspension period.
After the suspension, the Board may reissue the revoked cert. of reg. upon request of the medtech.
Section 27 – Foreign reciprocity
Foreign nationals can be licensed if their country allows Filipinos to practice Medical Technology
Section 28 – Roster of medical technologists
Prepared annually by Secretary of the Board
Includes name, address, citizenship, date of registration
Open to public inspection
Copies shall be mailed to each person
Placed on file in the Office of the President
All Department Heads and all agencies, offices
Instrumentalities of the Department of Health
and to such other offices, private or
governmental, and to the public) upon request
Section 29 – Penal provisions
Penalties: Not less than ₱2,000–and not greater than ₱5,000 or 6 months–2 years prison for:
Without being registered or exempted from registration
Even fully registered, practicing without supervision of a qualified personnel
Fraudulent reports
Refusing to display certificate
Using someone else's certificate
Impersonate any registrant of a fake or same name
Shall give any false or fraudulent device of any kind to the
Board of any member
Shall attempt to use a revoked or suspended certificate of registration
Use or advertise any title or description tending to
convey the impression that he is a Medical Technologist
without holding a valid certificate of registration
Violating Board rules or RA 5527 provisions
Who shall violate the rules and regulations of Board or orders after being approved and issued
Section 30 – Separability clause
If there’s any rule or regulation in this act that is declared invalid by a court of competent jurisdiction, the remainder of this Act shall not be affected by such declaration
Section 31 – Repealing clause
If there’s any inconsistency in this law then that is considered as invalid, but nothing in this act should be interpreted as invalid or amending any portion of the Medical Act of 1959
Section 32 – Effectivity clause
This Act shall take effect upon its approval.
Approved: June 21, 1969