3.2 Physical Chemistry
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
76 Terms
What is enthalpy change (ΔH)? (2)
- The heat energy transferred in a reaction
- Measured in kJ mol⁻¹
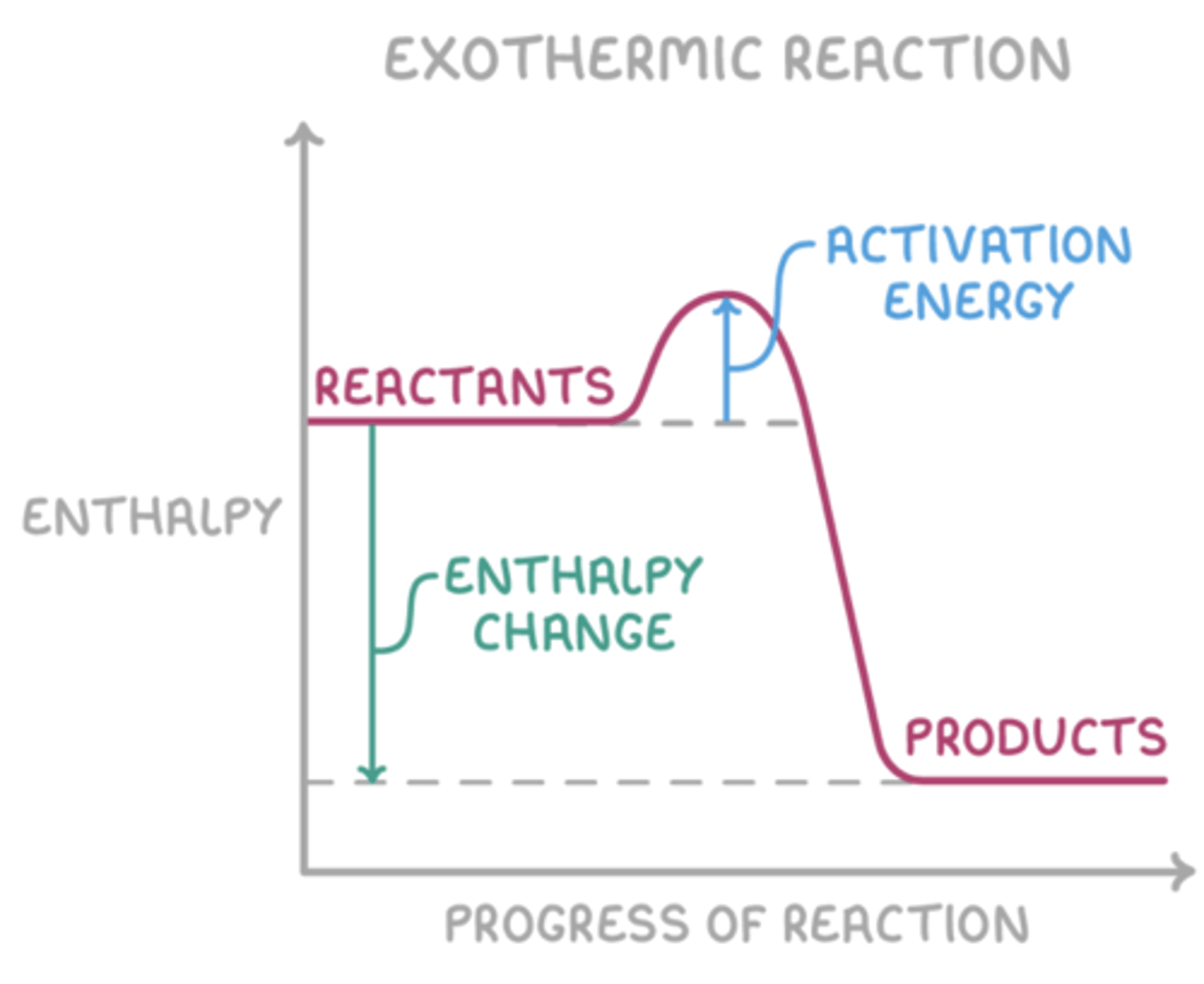
What happens in an exothermic reaction? (3)
- Energy is transferred from the system to the surroundings.
- The temperature of the surroundings increases.
- ΔH is negative.
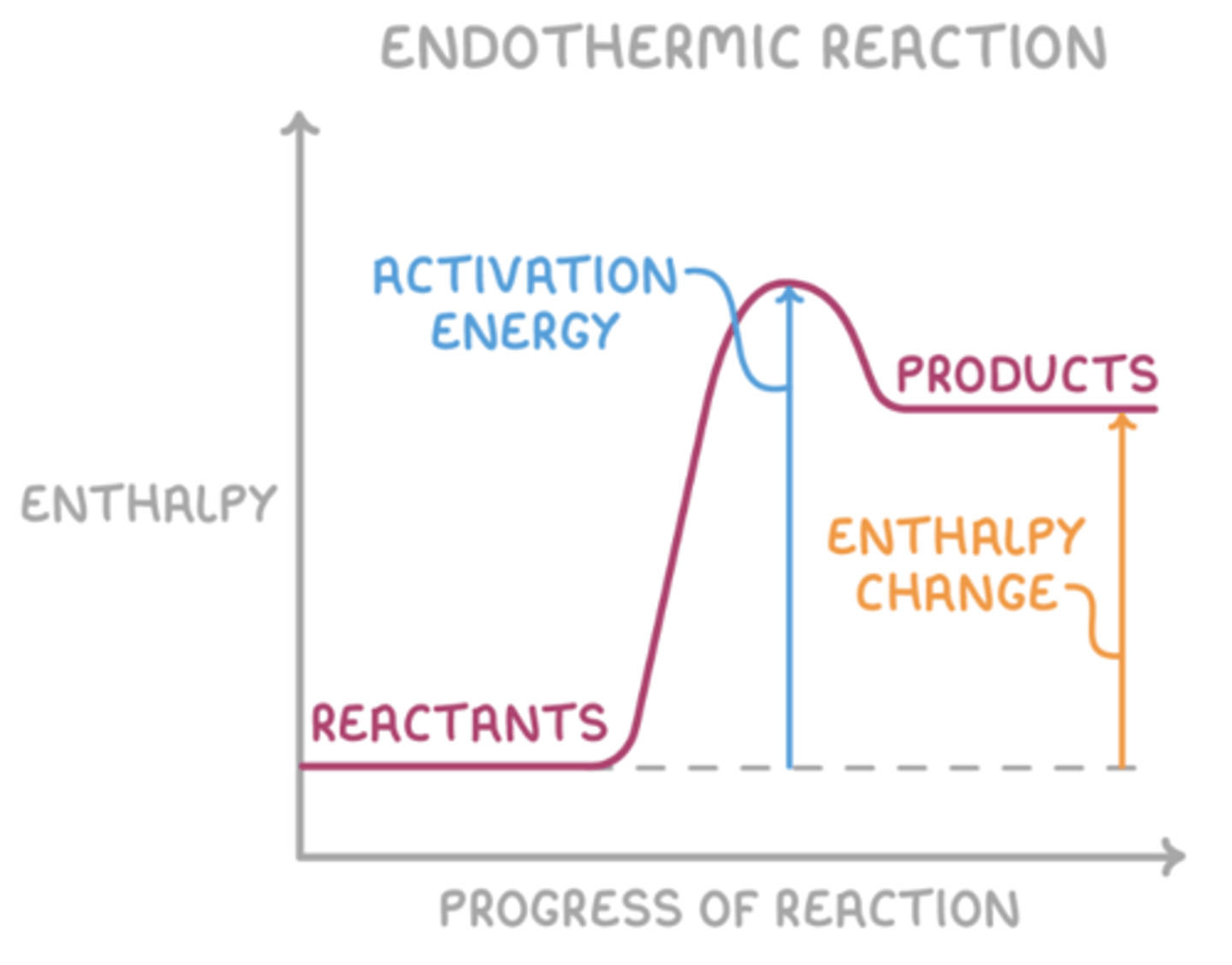
What happens in an endothermic reaction? (3)
- Energy is transferred from the surroundings to the system.
- The temperature of the surroundings decreases.
- ΔH is positive.
What is activation energy? (1)
The minimum energy required for a reaction to occur.
Draw an energy profile diagram for an exothermic reaction (4)
Draw an energy profile diagram for an endothermic reaction (4)
What are the standard conditions for a reaction? (4)
- 100kPa
- 298K
- 1 mol / dm^3
- Substances in their standard states
What is the standard enthalpy change of reaction (ΔᵣH°)? (3)
- The enthalpy change when a reaction occurs in the molar quantities shown in the chemical equation,
- with reactants and products in their standard states
- under standard conditions.
What is the standard enthalpy change of formation (ΔfH°)? (4)
- The enthalpy change when 1 mole of a compound is formed
- from its constituent elements
- in their standard states,
- under standard conditions.
What is the standard enthalpy change of combustion (ΔₐH°)? (4)
- The enthalpy change when 1 mole of a substance is completely burnt
- in excess oxygen,
- under standard conditions
- with reactants and products in their standard states.
What is the standard enthalpy change of neutralisation (ΔₙₑᵤₜH°)? (4)
- The enthalpy change when an acid and an alkali react together
- to form 1 mole of water,
under standard conditions
with reactants and products in their standard states.
What is the formula to calculate the energy transferred (q) in a reaction using calorimetry? (1)
q = mcΔT
What does m represent in the formula for q? (1)
mass (g)
What does c represent in the formula for q? (1)
Specific heat capacity ( J/gK)
What is the specific heat capacity of water? (1)
c - 4.18 J/gK
What does ΔT represent in the formula for q? (1)
Temperature change ( °C or K )
What is the formula for calculating enthalpy change (ΔH)? (1)
ΔH = -q / n
Why is q negative in the ΔH formula? (1)
Because the reaction is exothermic.
How do you determine the number of moles for different reactions using calorimetry? (2)
- Combustion: Use the moles of compound burnt.
- Neutralisation: Use the moles of water.
What are some common errors in calorimetry experiments? (4)
- Not standard conditions
- Not enough O2, resulting in incomplete combustion
- Alcohol evaporate off the wick
- Heat loss to other surroundings
How can incomplete combustion be avoided in calorimetry? (1)
- Use a source of pure O2
How can heat loss be avoided in calorimetry? (2)
- Use a lid on the calorimeter
- Use insulation around the calorimeter
What is average bond enthalpy? (2)
The energy required to break 1 mole of a specified type of bond
- in a gaseous molecule.
What is a limitation of average bond enthalpy values? (1)
Actual bond enthalpy varies depending on the chemical environment of the bond.
What type of reaction is bond breaking? (1)
Endothermic (requires energy)
What type of reaction is bond forming? (1)
Exothermic (releases energy)
How can enthalpy changes be calculated using average bond enthalpies? (2)
- Determine the number of types of bond in each reactant and product molecule
- ΔH = Σ(bond enthalpies reactants) - Σ(bond enthalpies products).
What is Hess's Law? (2)
- A reaction can take place by more than one route
- the total enthalpy change is the same for each route.
How do you calculate enthalpy change using enthalpy of formation data? (1)
ΔfH° = ΣΔfH° (products) - ΣΔfH° (reactants)
How do you calculate enthalpy change using enthalpy of combustion data? (1)
ΔcH° = ΣΔcH° (reactants) - ΣΔcH° (products)
How can enthalpy changes be calculated directly? (1)
Using calorimetry
How can enthalpy changes be calculated indirectly? (1)
Using Hess' Law
What is the rate of reaction? (1)
The change in a physical quantity over time.
What is the change in mass method for measuring reaction rate? (4)
- Place a conical flask on a balance and add the reactant.
- Measure the initial mass,
- Record the mass every 10 seconds until it stops changing.
- The decrease in mass is due to gas escaping.
What is the change in volume method for measuring reaction rate? (2)
- Collect and measure the volume of gas produced using a gas syringe or over water.
- Record the gas volume every 10 seconds until it stops changing.
How do you determine the rate of reaction at a specific time? (3)
- Create a graph from the data collected.
- Draw a tangent to the curve at the specific time
- The gradient of the tangent = the rate at that specific time.
What are the conditions for a successful collision in simple collision theory? (3)
- Reactants must collide
- with energy greater than the activation energy
- and in the correct orientation.
How does increasing concentration affect the rate of reaction? (2)
- Increases rate of reaction because more particles per unit volume.
- Increases frequency of successful collisions.
How does increasing pressure affect the rate of reaction? (2)
- Increases rate of reaction (only for gases).
- More particles per volume, leading to more frequent successful collisions.
How does increasing surface area affect the rate of reaction? (2)
- Increases rate of reaction as more particles are exposed.
- More particles with correct orientation, increasing successful collisions.
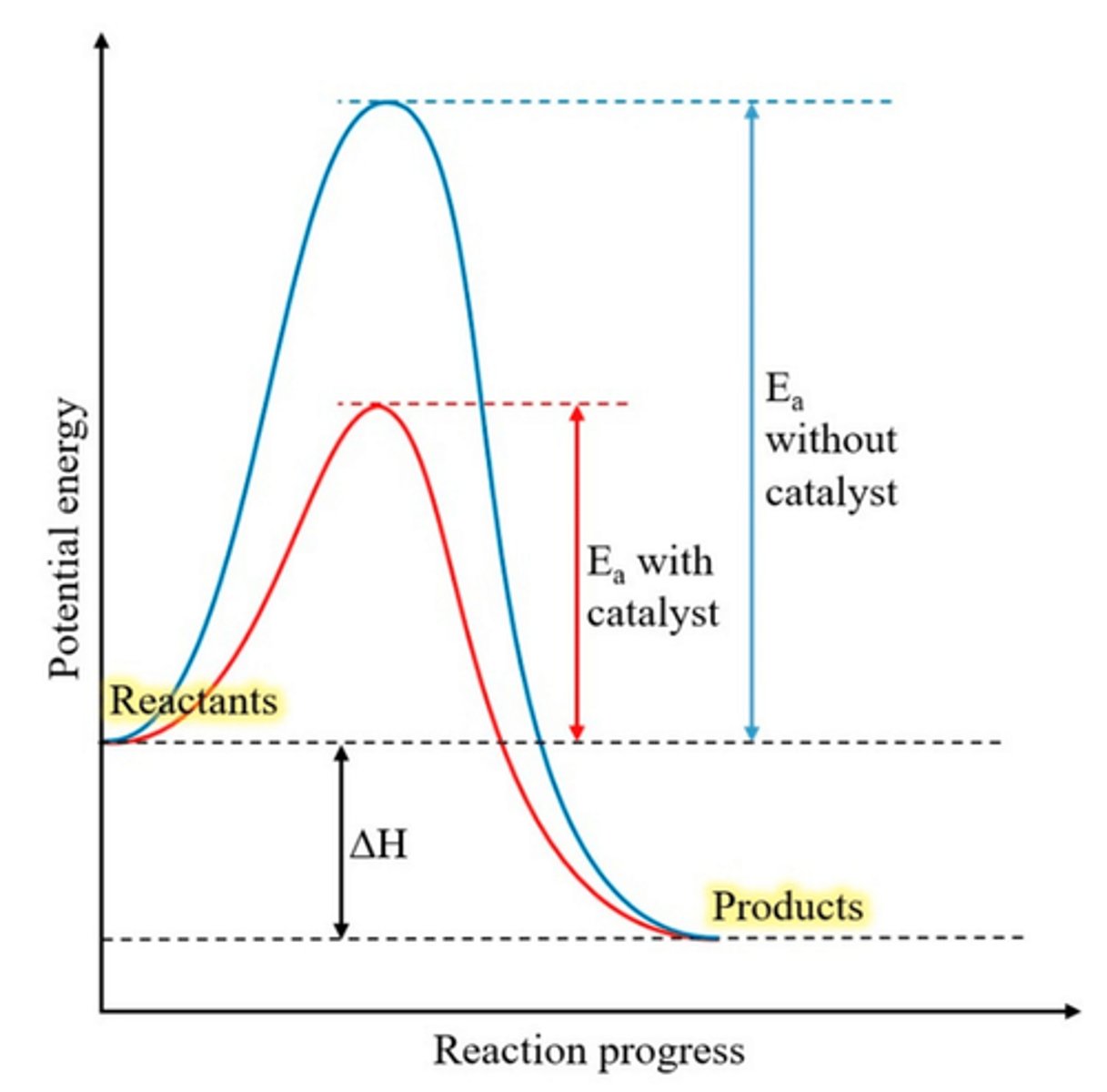
What is a catalyst? (3)
- A species that increases the rate of reaction
- by allowing the reaction to proceed via an alternative route with lower activation energy
- and is not used up in the reaction
What is a homogeneous catalyst? (1)
A catalyst in the same physical state as the reactants.
What is a heterogeneous catalyst? (1)
A catalyst in a different physical state from the reactants.
What are the benefits of using catalysts? (3)
- Reactions can happen with lower energy demands, reducing fossil fuel use.
- Less CO₂ is released, helping to reduce environmental impact.
- Lower costs due to reduced energy requirements.
How do catalysts improve atom economy? (2)
- They allow different reaction pathways to be used
- with a higher atom economy
Draw an energy profile for a reaction with and without a catalyst. (4)
Draw a model for the Boltzmann distribution. (4)
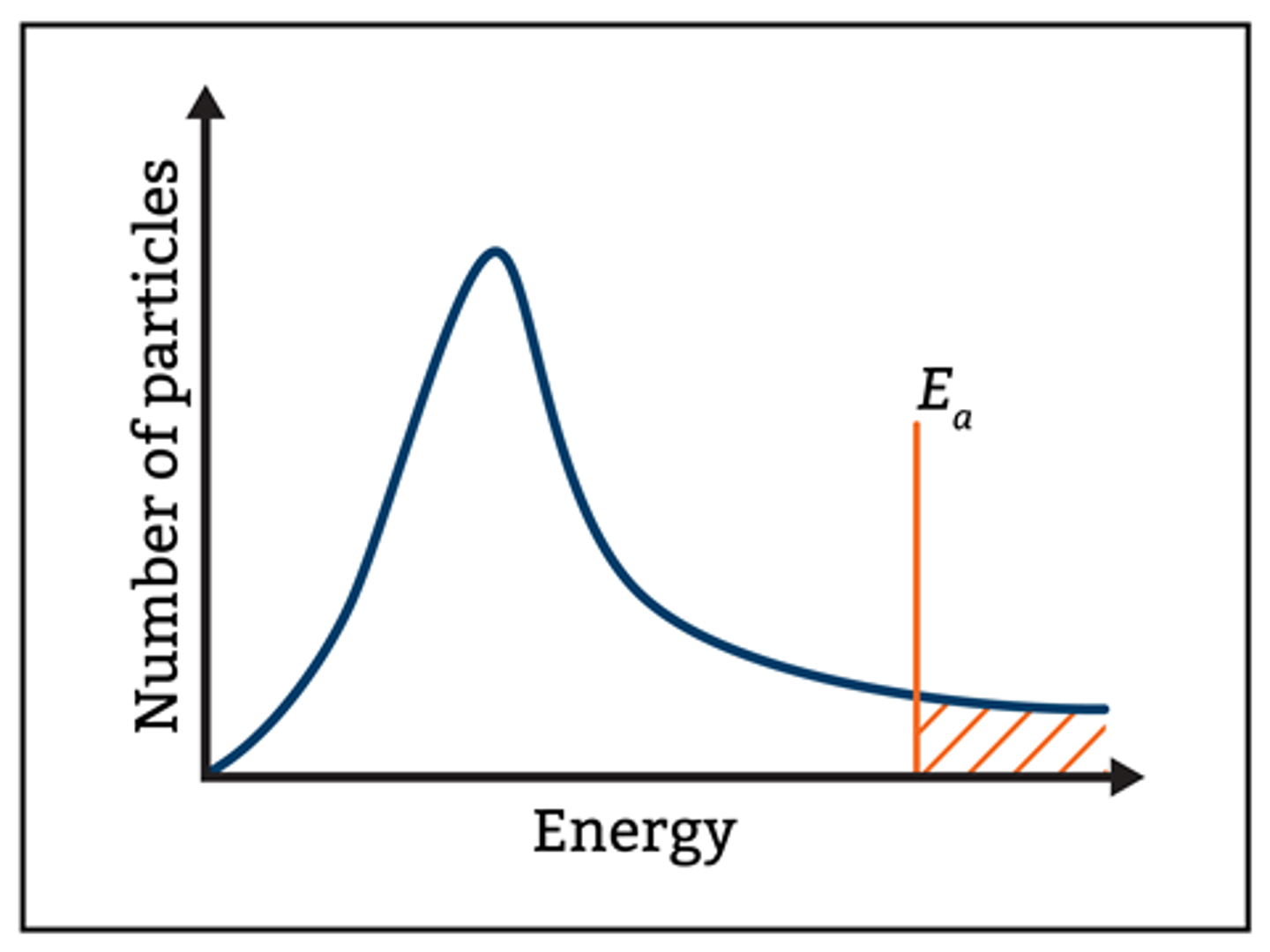
What does the Boltzmann distribution show? (1)
The distribution of energy among molecules in a sample.
Where is the most probable energy on a Boltzmann distribution graph? (1)
- The peak of the curve,
- where most molecules have low energy.
What does the area under the Boltzmann distribution curve represent? (1)
The total number of molecules in the sample.
Why do no molecules have zero energy in the Boltzmann distribution? (1)
Because all molecules have some energy due to constant motion.
Which molecules are able to react according to the Boltzmann distribution? (1)
Only molecules with energy greater than the activation energy (Ea) are able to react.
Draw a sketch of the Boltzmann distribution at two temperatures. (5)
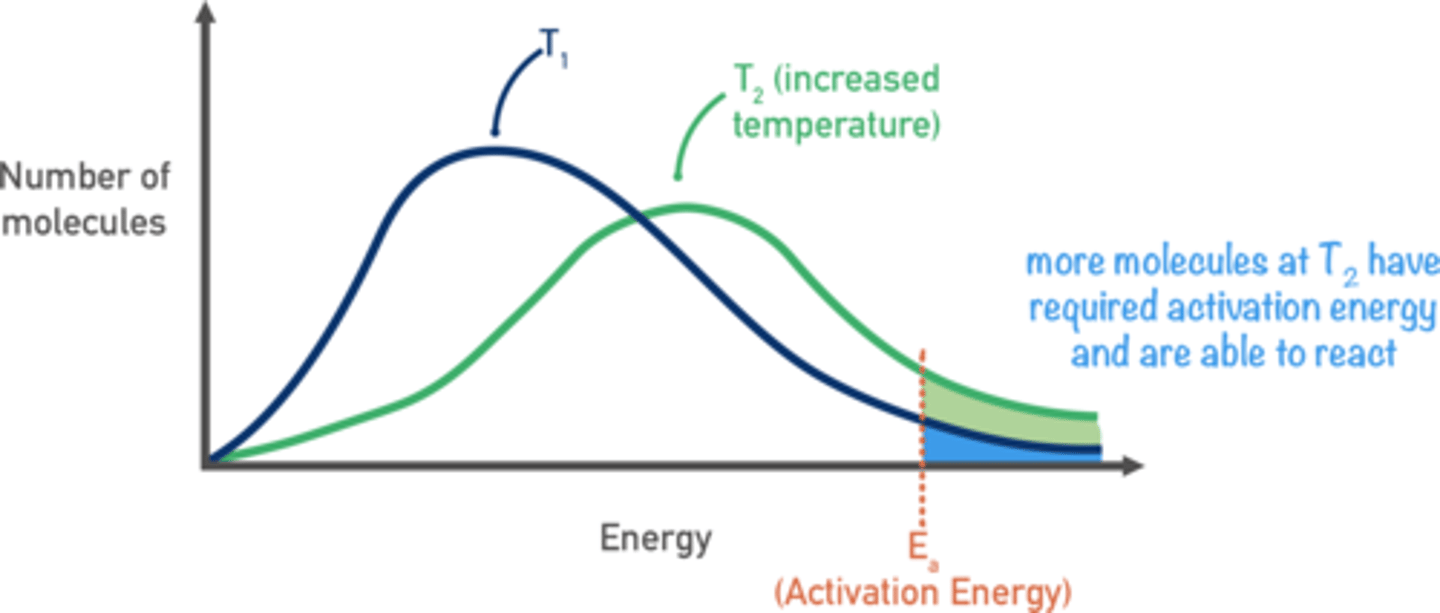
How does temperature affect the Boltzmann distribution? (2)
- As temperature increases, the curve flattens
- and shifts to the right.
How does increasing temperature affect the rate of reaction? (3)
- The kinetic energy of molecules increases.
- More molecules have energy greater than activation energy (Ea),
- leading to more successful collisions.
Draw a sketch of the Boltzmann distribution with and without a catalyst. (5)
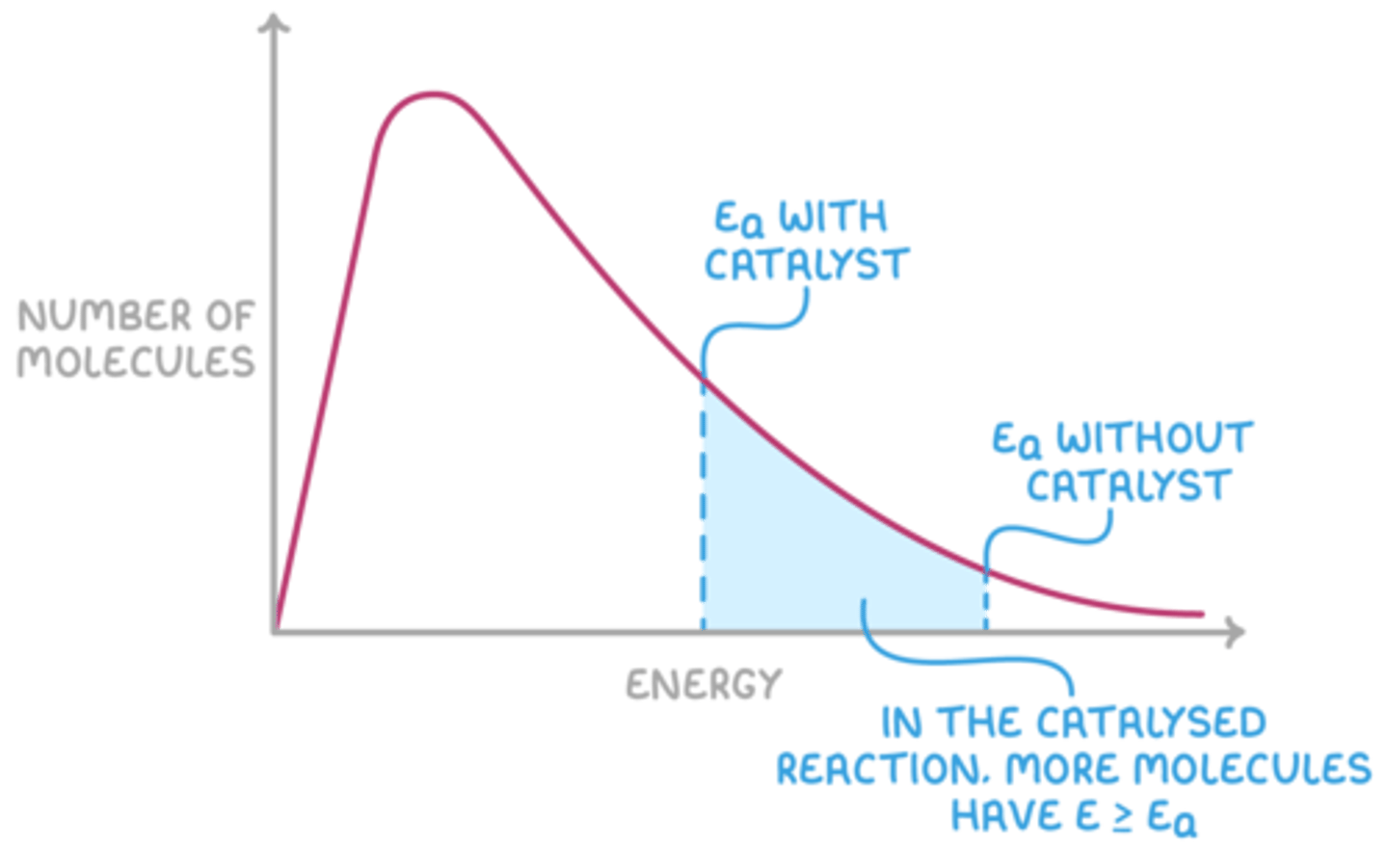
How does a catalyst affect the Boltzmann distribution? (1)
A catalyst does not change the energy distribution of molecules.
How does a catalyst increase the rate of reaction? (3)
- A catalyst lowers the activation energy (Ea).
- More molecules have energy greater than the new Ea,
- leading to more successful collisions.
What are the requirements for dynamic equilibrium? (3)
- The rate of the forward reaction is equal to the rate of the reverse reaction.
- The system must be closed with constant concentration of reactants and products,
- and unchanged reaction conditions.
What happens in a reaction at dynamic equilibrium? (2)
- Both forward and reverse reactions occur simultaneously
- with no observable change.
What is le Chatelier's principle?
- if a change is made to a system in dynamic equilibrium,
- the position of the equilibrium moves to counteract this change
How does increasing temperature affect equilibrium? (2)
- The equilibrium shifts in the endothermic direction.
- Helps to decrease the temperature.
How does increasing the concentration of reactants affect equilibrium? (2)
- The equilibrium shifts to produce more products.
- Helps to decrease the concentration of reactants.
How does increasing pressure affect equilibrium? (2)
- The equilibrium shifts to the side with fewer moles of gas.
- Helps to decrease the pressure.
How does a catalyst affect equilibrium? (3)
- No effect on the position of equilibrium.
- It increases the rate of both the forward and reverse reactions equally,
- making equilibrium reached faster.
Why must reaction conditions be realistic in the chemical industry? (3)
- To ensure an acceptable yield
- while maintaining a realistic rate of reaction.
- Factors like cost, safety, and environmental impact must be considered.
What are the conditions for the Haber process? (3)
- Temperature: 450°C
- Pressure: 200 atm
- Catalyst: Iron
What does the equilibrium constant (Kc) show? (1)
- The extent to which equilibrium has shifted
- by comparing the concentrations of products and reactants.
What is the equation for Kc? (1)
Kc = [products]ⁿ / [reactants]ⁿ
What do the square brackets [ ] represent in the Kc calculation? (1)
Square brackets indicate the concentration of the species (mol / dm^3)
What does the n represent in the Kc calculation? (1)
n = the mole ratio of the reactant / product
What does a value of Kc > 1 represent in the position of the equilibrium? (2)
- Reaction favours the products
- Equilibrium lies to the right
What does a value of Kc = 1 represent in the position of the equilibrium? (2)
- Neither reactants nor products are favoured
- The equilibrium is balanced
What does a value of Kc < 1 represent in the position of the equilibrium? (2)
- Reaction favours the reactants
- Equilibrium lies to the left
What physical property affects the value of Kc? (1)
Temperature only
What type of system can Kc values be calculated for? (2)
- Homogenous systems
- In equilibrium